
Conclusion: The results of this study indicate that low-intensity pulsed ultrasound (LIPUS) treatment at different frequencies can significantly improve erectile hardness and sexual satisfaction in patients with mild to moderate erectile dysfunction (ED). The treatment groups showed IIEF scores of 75% and 72.5%, respectively, and the effects were maintained one month after the final treatment.

Reference: DOI:10.13463/j.cnki.cczyy.2022.04.0024
In 2022, an article titled "Low-Intensity Pulsed Ultrasound Treatment for Mild to Moderate Erectile Dysfunction at Different Frequencies" was published in the Journal of Changchun University of Chinese Medicine (Vol. 38, Issue 4). This study, based on previous research, observed the clinical efficacy of LIPUS at different frequencies for treating mild to moderate ED and assessed its clinical effects one month after the last treatment, with follow-up satisfaction rates during the six months post-treatment.
Background
Erectile dysfunction (ED) is a common form of sexual dysfunction in men. According to the AUA guidelines, ED is defined as the persistent or recurrent inability to achieve and/or maintain an erection sufficient for sexual intercourse. Currently, the first-line treatment for mild to moderate ED is phosphodiesterase type 5 inhibitors (PDE5i), which benefit most patients. However, a small group of individuals are unresponsive to this treatment, and second-line treatments such as intracavernous injections only provide short-term relief and cannot reverse the pathological changes of ED. Previous studies suggest that LIPUS may become an important treatment for reversing the pathological changes of the penis in mild to moderate ED patients. However, its clinical application is still in the early stages, and its treatment duration, long-term efficacy, and safety remain unclear, necessitating further research.
Objective of the Study
To observe the clinical efficacy of low-intensity pulsed ultrasound (LIPUS) at different frequencies in treating male mild to moderate erectile dysfunction (ED).
Product Features
WBL LIPUS is a low-intensity pulsed ultrasound therapy. Its advantages include pulse design: low-intensity pulsed ultrasound (LIPUS) significantly reduces the thermal effects of continuous ultrasound therapy devices, optimizing the mechanical effects; non-invasive treatment: no invasive procedures are required, reducing patient discomfort and risk.
Biological Mechanism
Low-intensity pulsed ultrasound (LIPUS) is a safe and effective treatment for mild to moderate ED. Its main mechanism is to activate dormant endogenous stem cells within the body's tissues through mechanical forces, regulating cell signaling pathways to repair damaged blood vessels and nerve matrices, ultimately restoring their physiological functions.
Study Participants
1) Aged 18–60 years
2) Mild to moderate ED (IIEF-5 score of 8–21)
3) ED duration of >3 months and<10 years
4) Healthy with stable sexual partner and sexual life
5) Not used PDE5i or other ED treatments in the past two weeks prior to enrollment
Treatment Method
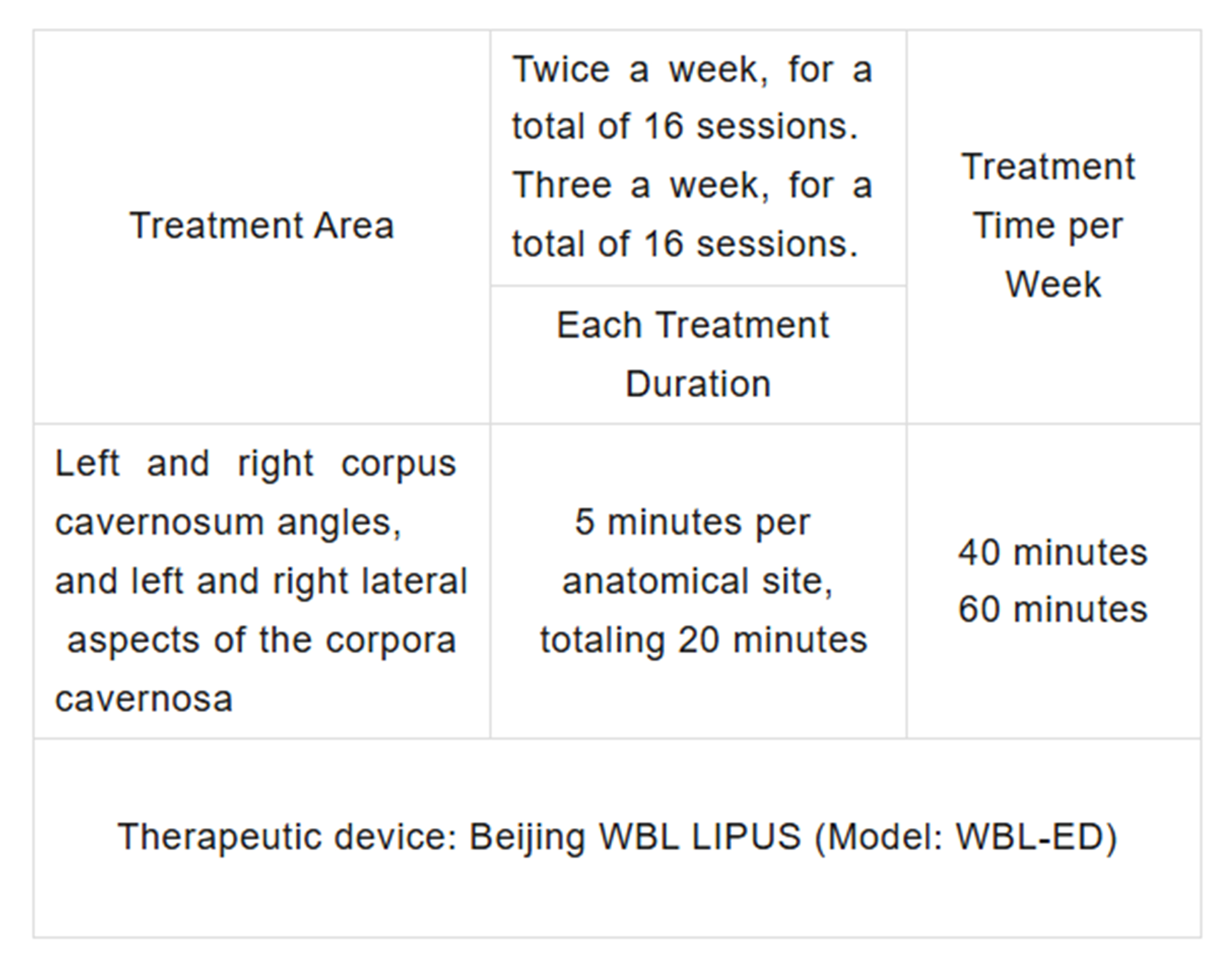
Treatment Area and Contact Method:
Patients received ultrasound therapy twice a week for 8 weeks (16 treatments total). Each treatment session involved four target areas.
The details of the treatment time and areas are outlined in the table below.
Observation Indicators
Primary evaluation index: IIEF-5 score. Secondary evaluation indices: Male erection hardness score (EHS), penile erection angle, sexual activity diary (SEP), and overall assessment questionnaire (GAQ), with treatment efficacy determined based on the minimum clinically significant difference (MCID) in IIEF-5 scores.
Study Results
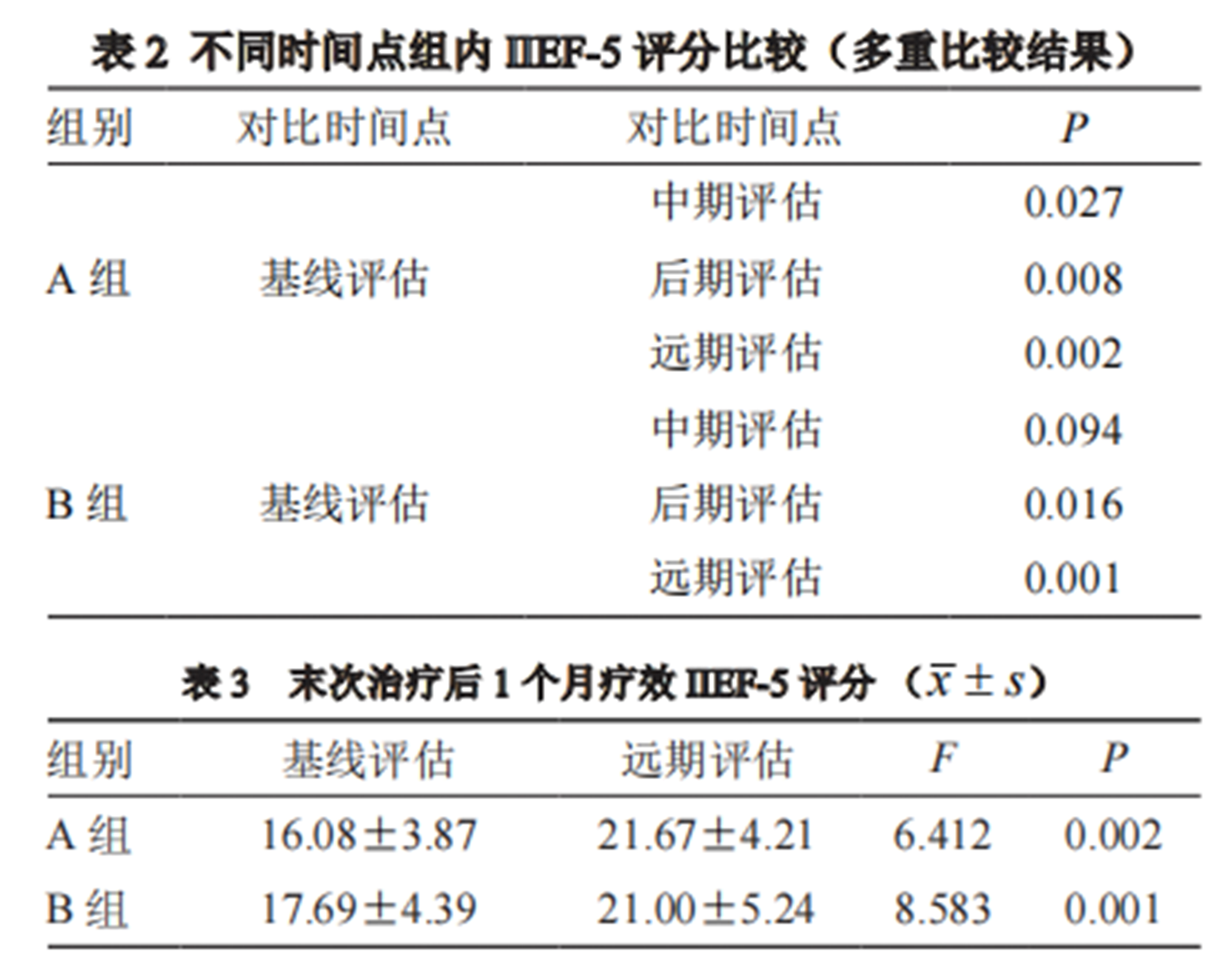
1. Compared to baseline, Group A showed a significant increase in IIEF-5 scores after the 8th treatment, while Group B showed no significant improvement until the 16th treatment. Both groups continued to show progressive increases in IIEF-5 scores as treatment progressed. Both groups showed significant improvement in IIEF-5 scores one month after the last treatment, with no significant difference compared to the final treatment.
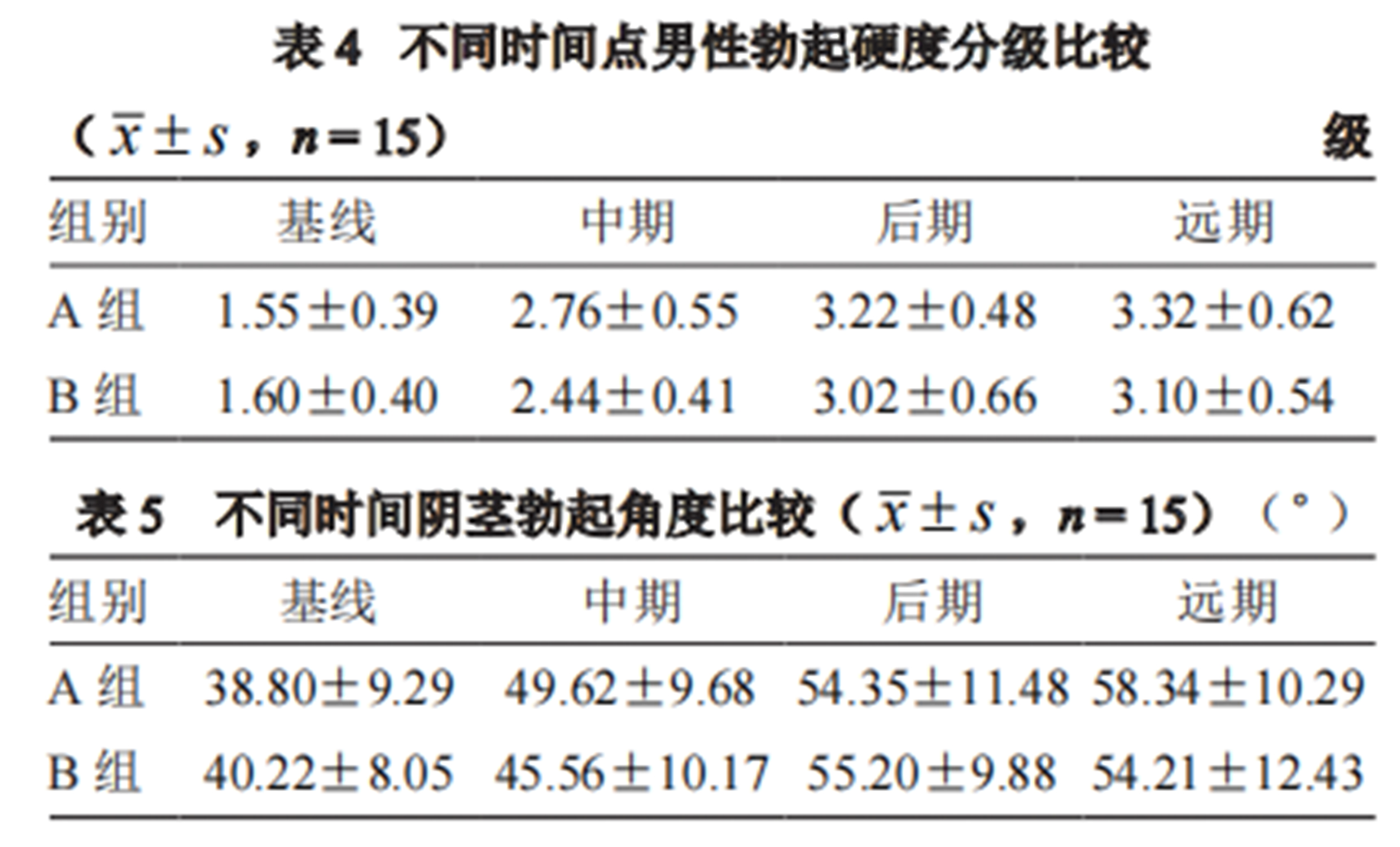
2. Both groups showed significant improvement in erection hardness scores and penile erection angles after treatment, with Group A achieving optimal results in a shorter time. Both groups maintained the effect one month after the last treatment, with no significant difference between groups.
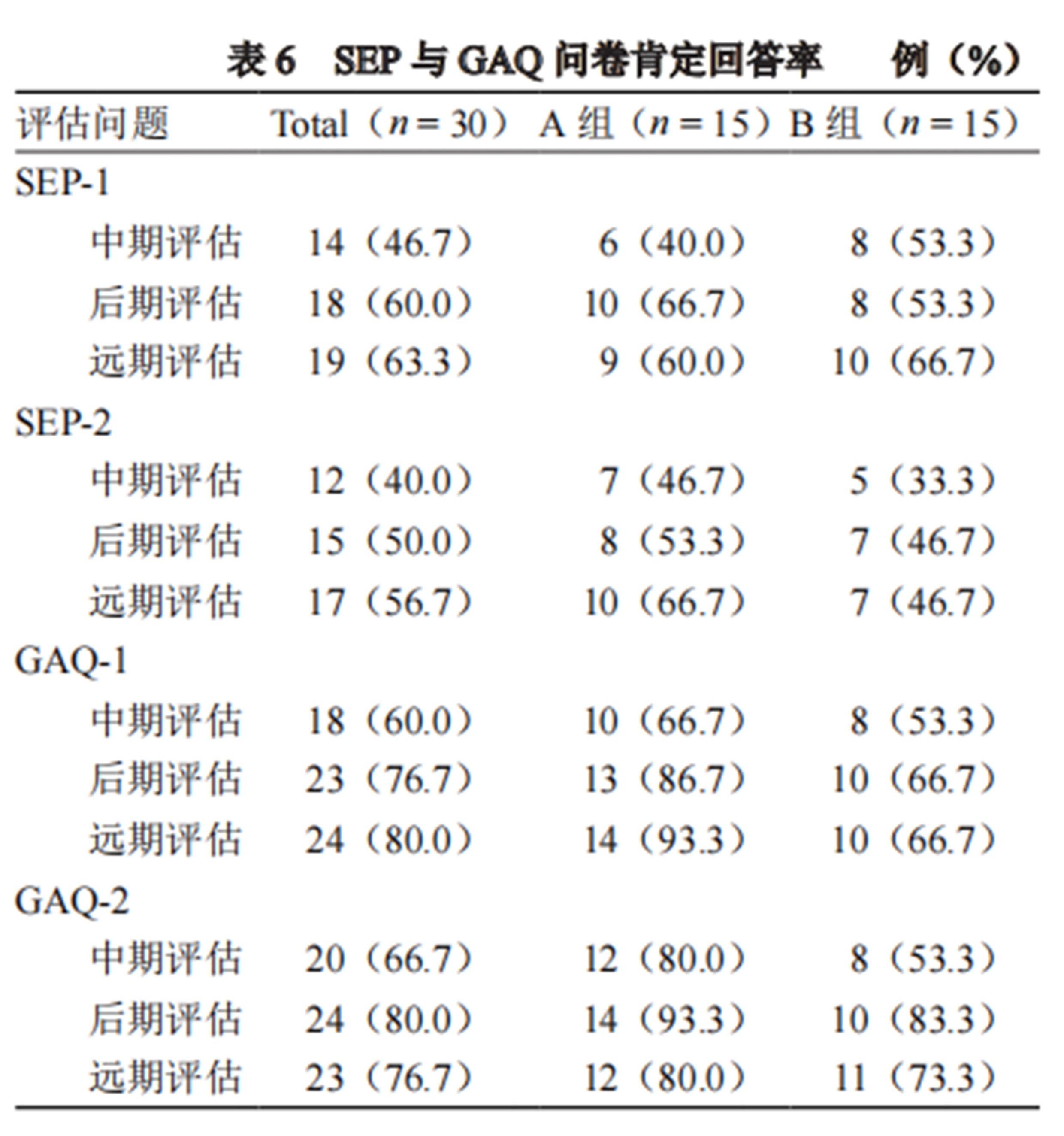
3. SEP and GAQ positive response rates were not significantly different between the two groups at the same treatment stage. However, both groups showed significant increases in SEP and GAQ positive response rates as treatment continued, with the GAQ positive response rate reaching 93.3%.
4. The overall treatment efficacy was evaluated using MCID: 75% for Group A and 72.5% for Group B at the end of treatment, with no significant difference. One month after treatment, the efficacy rates for the two groups were 73.6% and 71.0%. No adverse reactions were reported during or after treatment.
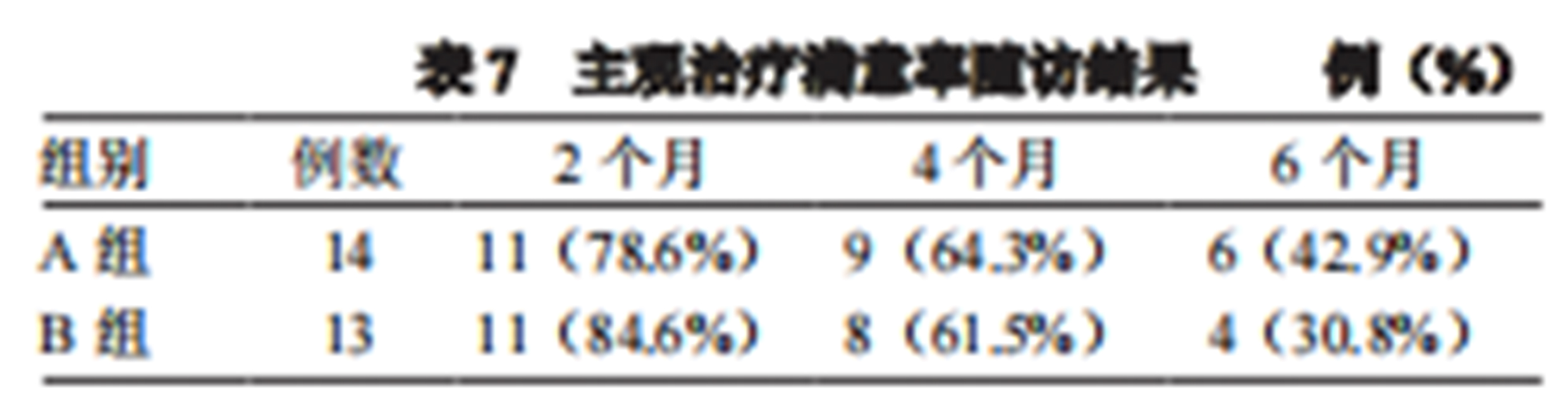
5.Follow-up surveys conducted at the 2nd, 4th, and 6th months after treatment, with 27 participants completing three telephone follow-ups, showed no significant difference in subjective treatment satisfaction between the two groups. The overall satisfaction rate was as high as 84.6% at 60 days. However, satisfaction declined over time, with overall subjective treatment satisfaction remaining above 60% for the first four months after treatment.
Conclusion
This study found that, one month after the final treatment, patients' efficacy scores, erection hardness, and erection angle were significantly improved and maintained at an optimal level. The treatment also improved sexual satisfaction, self-confidence, and self-esteem in mild to moderate ED patients. However, telephone follow-up showed that satisfaction declined over time, reaching 60% or higher in the first four months post-treatment. These results provide clinical reference for determining the duration of the next treatment cycle. The treatment process was free from adverse events such as burning, pain, injury, inflammation, or infection. LIPUS treatment for mild to moderate ED demonstrates good long-term efficacy and safety, and it has the potential to become an important treatment option for mild to moderate ED in clinical practice.

The treatment device used in this trial was the WBL LIPUS Model WBL-ED.
WBL Medical Equipment Co., Ltd. is a pioneer in LIPUS technology. With its independently developed LIPUS-ED device, the company was the first to apply low-intensity pulsed ultrasound technology to treat erectile dysfunction, providing a non-invasive, safe, and effective solution. Since receiving approval from the China Food and Drug Administration (CFDA) in 2018, the WBL LIPUS-ED device has filled a technological gap in this field both domestically and internationally, earning recognition as an innovative medical product and demonstrating WBL's industry leadership and technological expertise.