

On June 16, 2025, the team led by Professor Fu-Tai Lu from the University of California, San Francisco (UCSF), officially signed a strategic cooperation agreement with the Shanghai Male Science Research Institute and the Department of Urology at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine. The signing ceremony and the "2025 China Urology and Andrology Innovation Development Forum" were held grandly in Shanghai. Guo Mao, Chairman of WBL Medical Group, attended the opening ceremony as a special guest and gave a speech sharing the group's innovative practices in medical technology and the forward-looking layout of the industrialization of andrology disease treatment technologies. Professor Fu-Tai Lu and Professor Lu Mu-Jun, Director of the Department of Urology at Ruijin Hospital, also delivered keynote speeches on multi-center clinical research design and the frontier theories of energy medicine, injecting academic energy into international cooperation.

Focus on Clinical Translation of LIPUS Technology


1. Multi-Center Clinical Research
Led by Professor Lu Mu-Jun, Director of the Department of Urology at Ruijin Hospital, and in collaboration with several top-tier hospitals in China, a multi-center randomized controlled trial (RCT) will be launched to investigate the effectiveness of LIPUS in treating chronic prostatitis/chronic pelvic pain syndrome (CP-CPPS). The research will strictly follow international clinical research protocols, enrolling patients from different stages and age groups, and using standardized evaluation systems to verify the efficacy and safety of the technology.

2. Mechanism and Theoretical Collaborative Research
Both parties will jointly explore the molecular biology mechanisms of LIPUS technology, focusing on its effects on the activation of endogenous stem cells and the regulation of the tissue microenvironment. Professor Fu-Tai Lu's theory of "activation of endogenous stem cells" provides the core framework for this cooperation. This theory, which involves precise physical energy interventions in cell signal transmission to stimulate the regenerative potential of autologous stem cells, aligns well with WBL Medical Group's technology development focus on "putting the patient's self-healing ability at the core."
Energy Medicine and Endogenous Stem Cell Activation


In his speech, Professor Fu-Tai Lu elaborated on the clinical value of this theory:
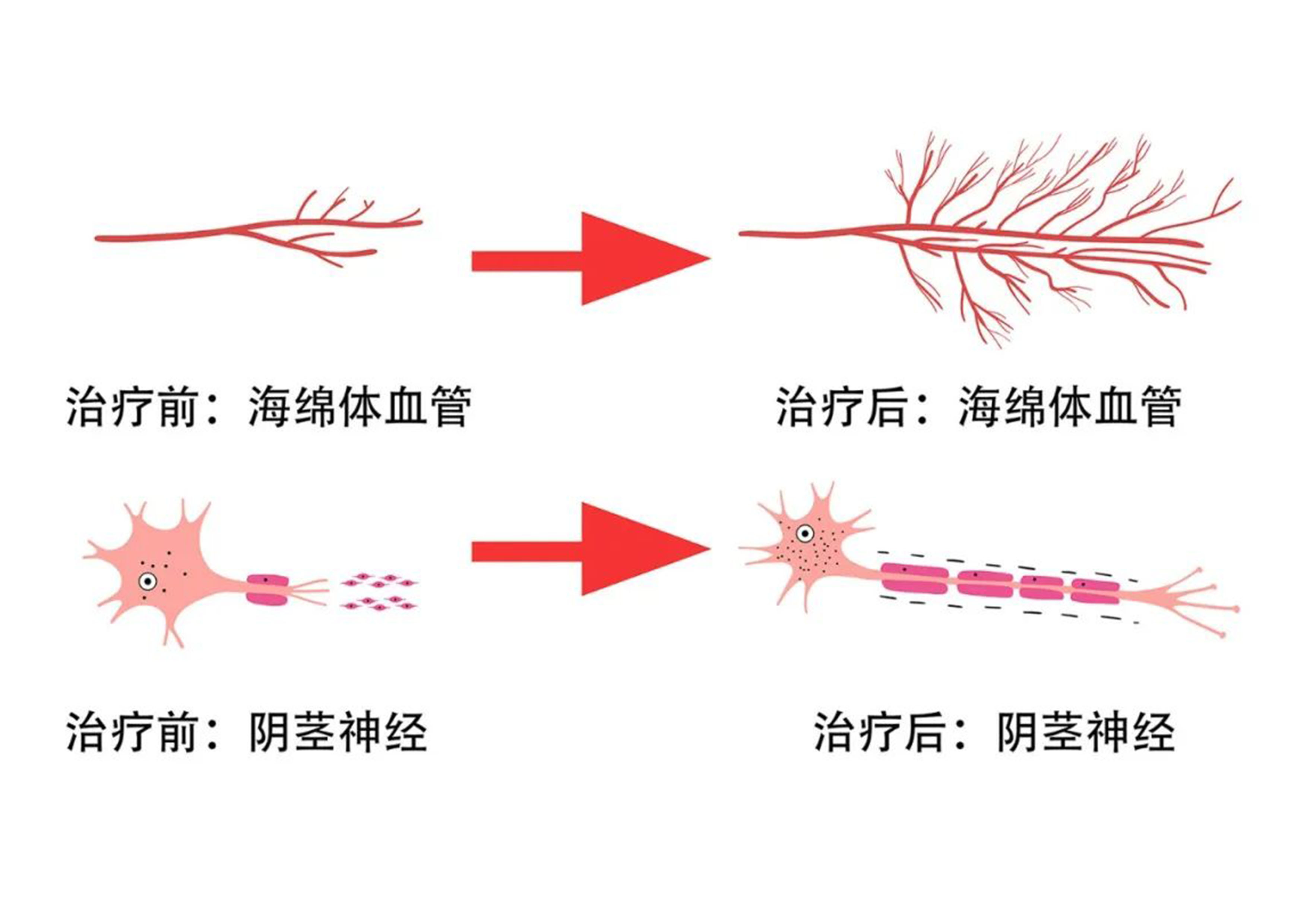
1、Energy Regulation Mechanism: Using physical energy such as ultrasound to precisely intervene in cell signal transmission, triggering the transition of autologous stem cells from a resting state to a proliferative and differentiated state, thus achieving tissue repair and regeneration.
2、Technological Differentiation Advantage: Compared to exogenous stem cell transplantation, the strategy of activating endogenous stem cells avoids the risk of immune rejection and relies on the body's own repair system, offering both safety and sustainability.
3、Clinical Application Prospects: In addition to CP-CPPS, this theory can also be extended to osteoarthritis, diabetic ulcers, and other fields, providing new paradigms for the treatment of chronic diseases.
Clinical Value of LIPUS Technology

WBL Medical Group’s LIPUS technology, as an original Chinese physical energy therapy device, has the following characteristics:
1、Non-invasive Treatment: It precisely targets the tissue through low-intensity pulsed ultrasound, enabling non-invasive intervention.
2、Independent Intellectual Property: The device development, treatment parameter design, and clinical protocol have all been completed by the WBL team, forming a complete technological closed loop.
3、Multi-modal Evaluation System: Combining stem cell marker detection, inflammatory factor analysis, and functional assessment to quantify treatment effects, providing scientific basis for clinical decision-making.
Scientific and Normative Balance
This multi-center clinical study will follow a rigorous design protocol:
1、Randomized Grouping: Patients will be randomly assigned to the LIPUS treatment group or the control group based on disease stage, age, and other factors.
2、Endpoint Indicators: The primary endpoint is pain scoring and improvement in quality of life; secondary endpoints include changes in stem cell markers, tissue repair levels, and more.
3、Long-Term Follow-up: A patient database will be established to track long-term treatment outcomes and safety, providing data support for optimizing the technology.
Deepening Industry-Academia-Research Collaboration



After the signing ceremony, Chairman Guo Mao, Professor Fu-Tai Lu, and Director Lu Mu-Jun visited the Shanghai Male Science Research Institute, where they focused on examining the clinical application scenarios of LIPUS technology and the establishment of the andrology disease sample library. The three parties discussed in-depth on the direction of technological iteration, data-sharing mechanisms for clinical research, and future commercialization paths, reaching a consensus on further collaboration. Chairman Guo Mao stated that WBL Medical Group will continue to promote deep integration of industry, academia, and research, helping Chinese original medical technology go global.
WBL LIPUS
The cooperation between UCSF and the Shanghai Male Science Research Institute reflects the deep integration of global academic resources and Chinese clinical practices. Through the clinical translation and theoretical innovation of WBL's LIPUS technology, both parties aim to provide safe and effective solutions for chronic urology diseases. In the future, as research deepens, the combination of energy medicine and regenerative medicine may bring breakthroughs in more disease areas, driving the global medical innovation process.

