
Conclusion: This study designed a comparison between Group A (3 times per week) and Group B (2 times per week). After 8 weeks of LIPUS treatment, Group A and Group B showed IIEF scores of 80.5% and 77.5%, respectively. No adverse reactions were reported in any of the cases. Both treatment frequencies of LIPUS were equally safe and effective for mild to moderate ED, with the 3-times-per-week treatment plan achieving good results in a shorter time.

Reference: Natl Med J China, May 12, 2020, Vol. 100, No. 18
In 2020, an article titled "Observation of the Effectiveness and Safety of Low-Intensity Pulsed Ultrasound (LIPUS) Mechanical Force at Different Frequencies in Treating Erectile Dysfunction" was published in the National Medical Journal of China, Vol. 100, No. 18, on May 12. The study was conducted from May to September 2019 at the Urology Department of a Medical Center with 40 patients diagnosed with mild to moderate ED. The patients were randomly divided into two groups: Group A (3 times per week) and Group B (2 times per week). Both groups underwent 16 sessions of LIPUS treatment with identical working parameters.
Research Background
Erectile dysfunction (ED) is a common condition among adult men, with an incidence of about 52% in men aged 40–70 and 22% in men under 40. It is estimated that by 2025, there will be more than 300 million ED patients globally. ED severely affects the quality of life of patients and their partners, and it may also be an early symptom and risk factor of cardiovascular diseases. The first-line treatment for ED is phosphodiesterase type 5 (PDE5) inhibitors, but the proportion of non-responders to these medications is 22–37%. Additionally, 2%–15% of patients discontinue treatment due to side effects. Penile prosthesis implantation can cure ED completely, but many patients avoid it due to the unavoidable surgical complications. Low-Intensity Pulsed Ultrasound (LIPUS) has recently emerged as a promising treatment for ED.
Research Objective
To observe the efficacy and safety of Low-Intensity Pulsed Ultrasound (LIPUS) mechanical force in treating erectile dysfunction (ED), and to evaluate the impact of different treatment frequencies on its effectiveness.
Product Features
WBL LIPUS is a type of low-intensity pulsed ultrasound therapy. Its advantages include: Pulsed Design: The low-intensity pulsed ultrasound (LIPUS) significantly reduces the thermal effects of continuous ultrasound treatments, optimizing the mechanical effects.Non-invasive Treatment: It does not require invasive procedures, reducing patient discomfort and risks.
Biological Mechanism
LIPUS induces and recruits stem cells' proliferation and differentiation, including penile stem/progenitor cells, multipotent stem cells derived from neural crest stem cells, adipose progenitor cells, and mesenchymal stem cells. The penis has endogenous penile stem/progenitor cells, and due to their powerful directional differentiation potential, under LIPUS mechanical force, these cells can differentiate into endothelial cells, smooth muscle cells, and neurons. This provides a foundation for repairing pathological changes in the erectile tissue of ED.
Study Participants
1.Age: 20–60 years
2.Overall health: Stable heterosexual partners for >3 months
3.ED duration: ≥3 months,<10 years
4.Mild to moderate ED with IIEF-EF scores of 11–25
7.No PDE5 inhibitors or other ED treatments used in the past weeks before grouping.
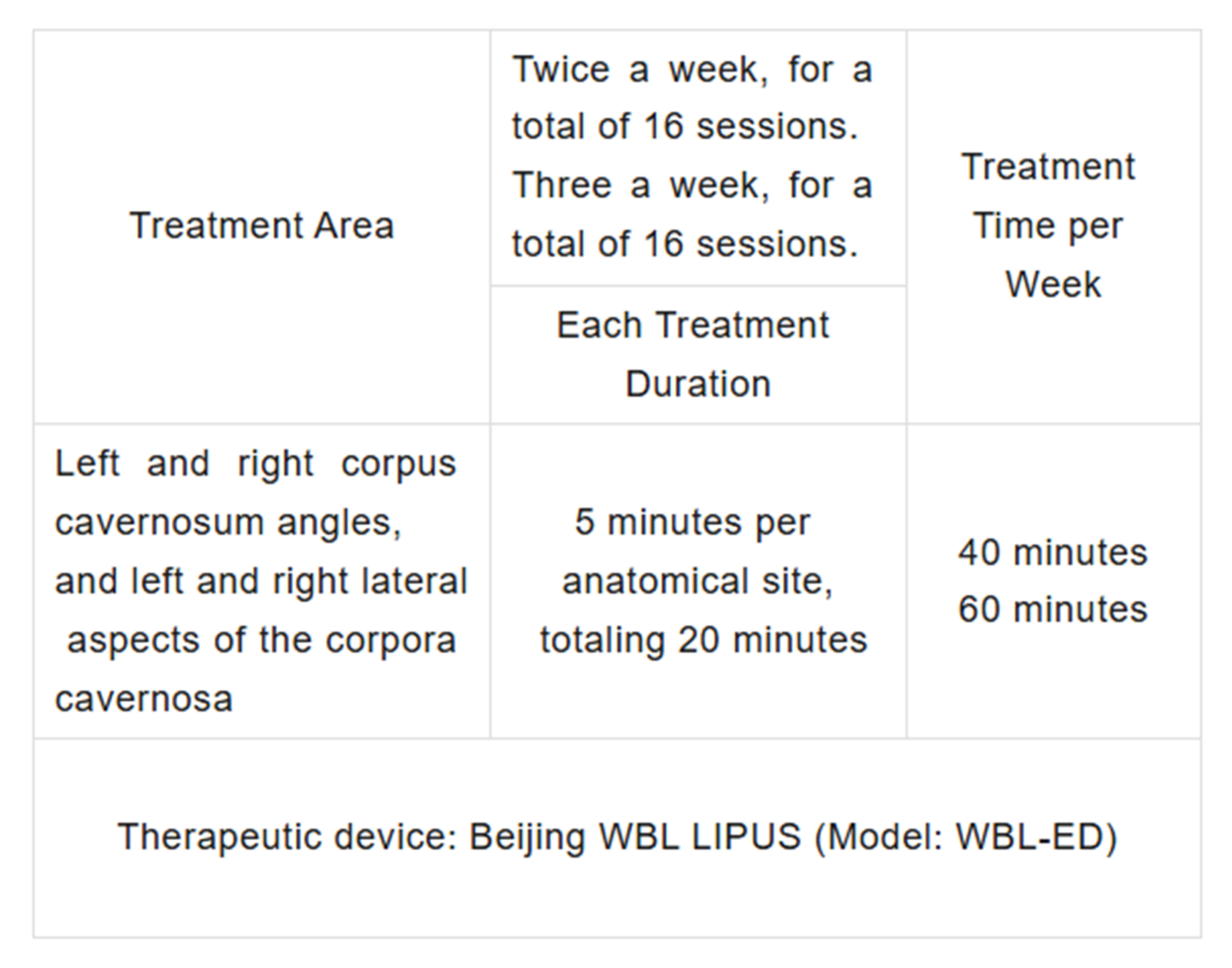
Treatment Method
Patients underwent ultrasound treatment twice per week for 8 weeks, totaling 16 sessions. Each session treated four areas. The treatment time and areas are shown in the following table:
Observation indicators
The primary efficacy assessment indicator is the IIEF-EF score. The secondary indicators include the Erectile Hardness Score (EHS), Self-Esteem and Sexual Relationship Questionnaire (SEAR), Sexual Activity Diary (SEP - Questions 2/3), and Overall Assessment Questionnaire (GAQ - Questions 1/2). Treatment effectiveness is determined based on the Minimum Clinically Important Difference (MCID): an increase of ≥2 points in the IIEF-EF score for mild ED, or an increase of ≥5 points in the IIEF-EF score for moderate or severe ED after treatment.
Study Results
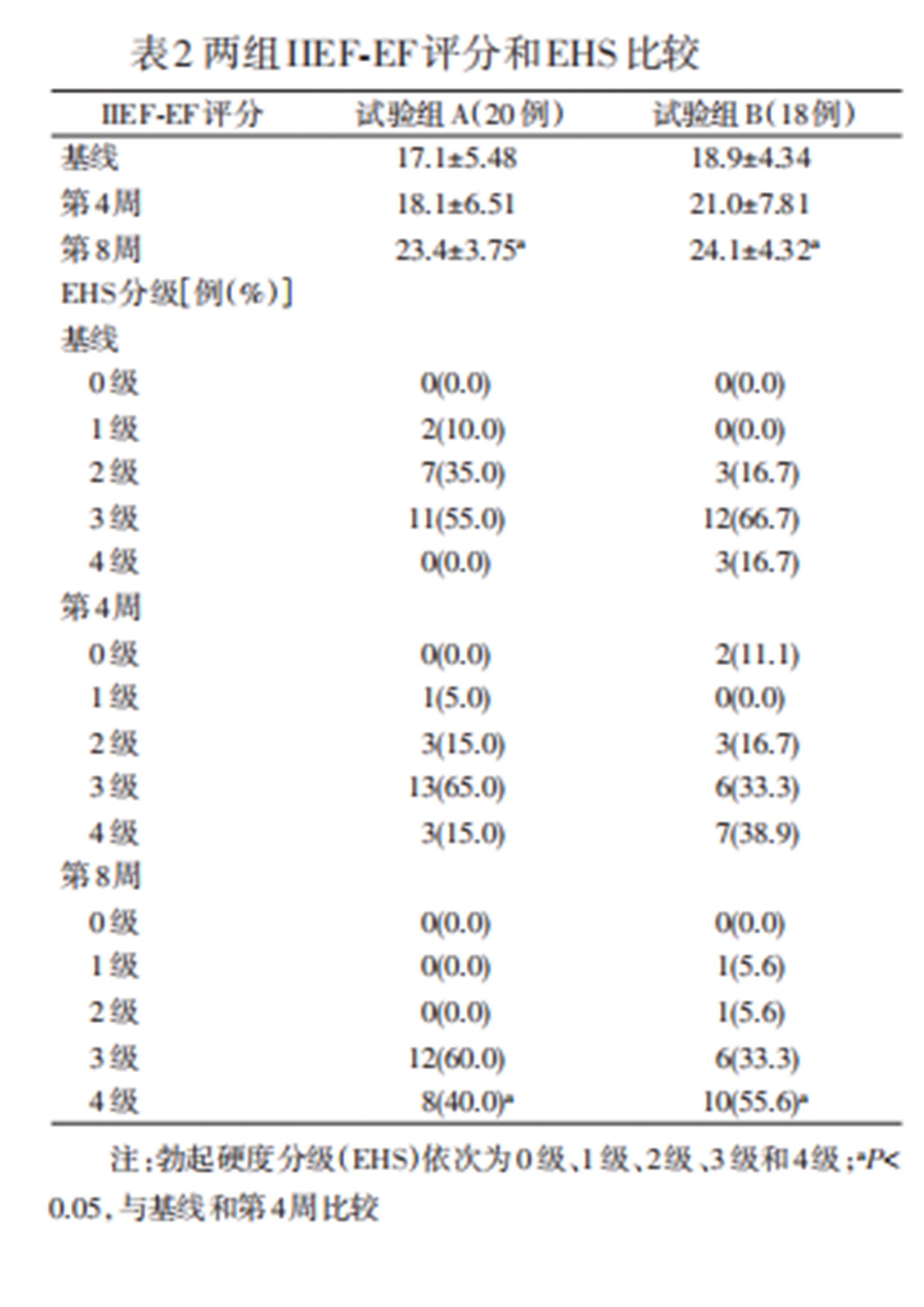
Compared to baseline and the 4th week (mid-term evaluation), both groups showed significant improvements in IIEF-EF scores by the 8th week (end evaluation). No significant statistical difference was found between Group A and Group B at baseline, the 4th week, and the 8th week. Similarly, both groups showed a significant increase in the proportion of 4-level erectile hardness by the 8th week, with statistically significant differences between baseline and the 4th week. No significant difference was observed between the two groups regarding EHS scores at baseline, the 4th week, and the 8th week.
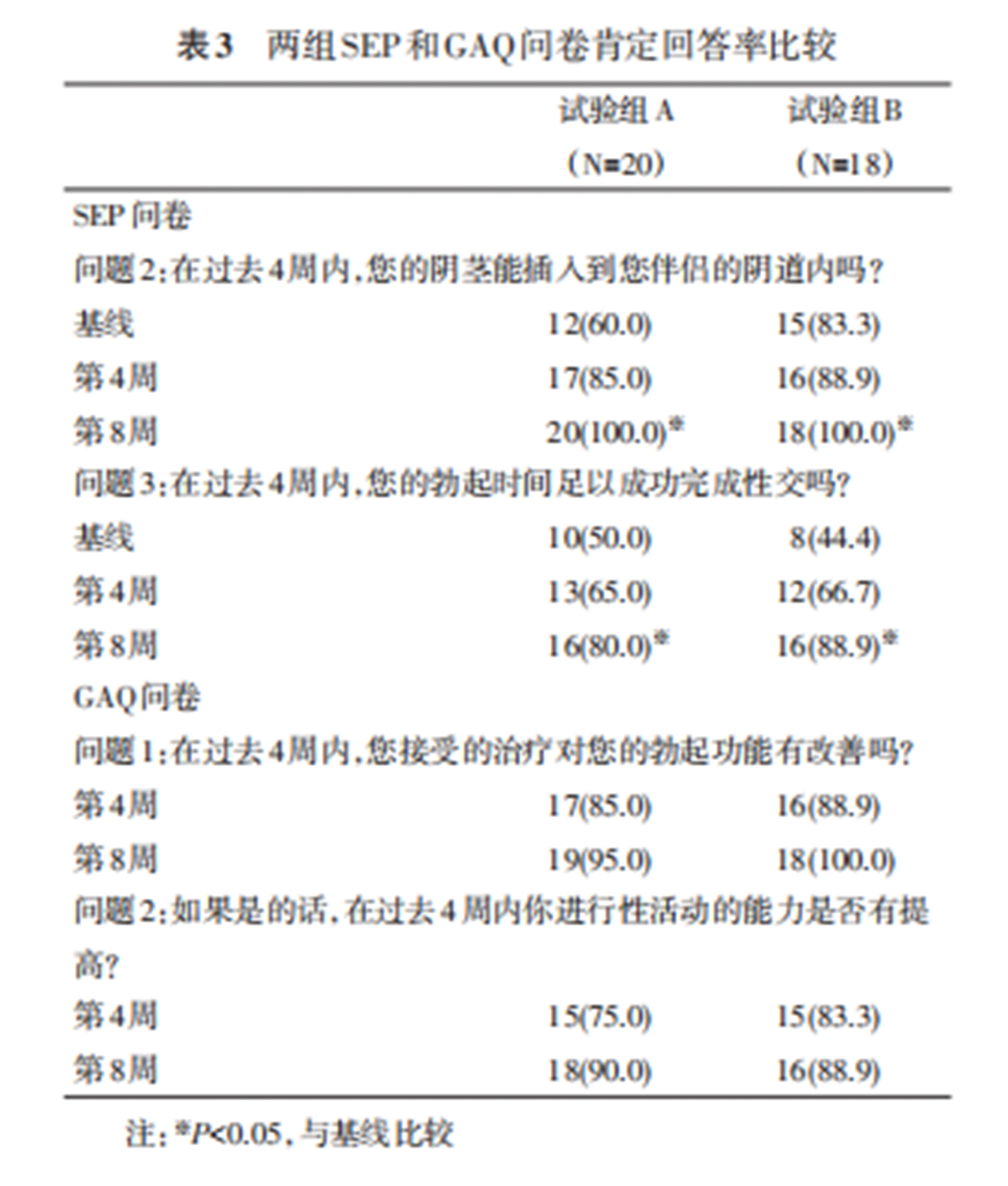
Both groups showed improvements in self-confidence and self-esteem scores by the 8th week, with significant improvement in sexual and general relationship scores. No significant difference was found between the two groups in SEAR scores at baseline, the 4th week, and the 8th week. Additionally, SEP affirmative response rates were significantly higher by the 8th week, with no statistical difference between the two groups at baseline, the 4th week, and the 8th week. By the 4th and 8th weeks, both groups had a GAQ affirmative response rate of over 75%, with no significant difference between the two groups.
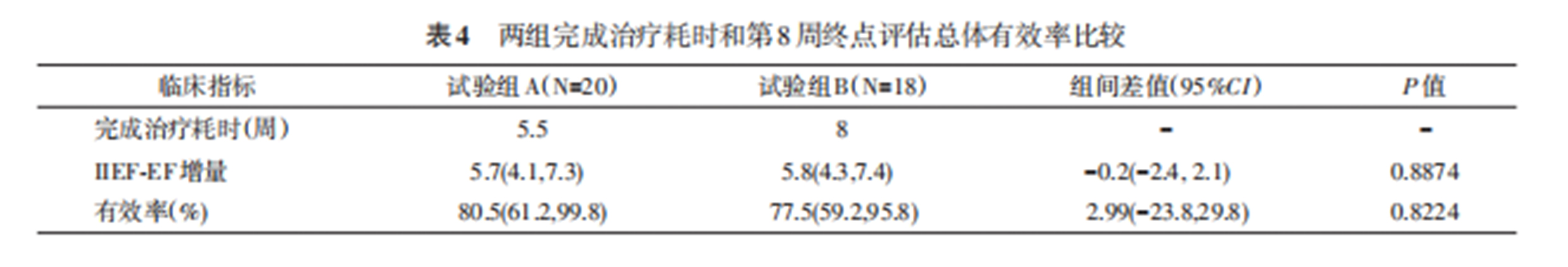
According to MCID, at the 8th-week final evaluation, IIEF-EF scores were 80.5% for Group A and 77.5% for Group B, with no significant difference. Group A completed treatment 2.5 weeks faster than Group B. The total number of treatments completed was 320 for Group A and 285 for Group B. No cases reported pain scores higher than 1, and no adverse reactions such as skin bruising, hematuria, or contusions were observed.
Research Conclusion
This study confirmed that both treatment frequencies of LIPUS are equally safe and effective for mild to moderate ED. The 3-times-per-week treatment plan achieves good results in a shorter period. However, long-term effects need further observation.
The treatment device used in this study was the WBL LIPUS model WBL-ED
WBL Medical Equipment Co., Ltd. is the pioneer of LIPUS technology. With its independently developed WBL-ED device, it was the first to apply low-intensity pulsed ultrasound technology to ED treatment, providing a non-invasive, safe, and efficient new solution. Since its approval by the China Food and Drug Administration (CFDA) in 2018, WBL LIPUS has filled a technical gap in this field and won the China National Medical Device Innovation Product Award, highlighting its leading position and technical strength in the industry.
