
Conclusion: The study results show that patients with Peyronie’s Disease (PD) treated with WBL low-intensity pulsed ultrasound (LIPUS) therapy for 4 weeks experienced significant reductions in pain symptoms, even to the point of disappearance. This suggests that early intervention can effectively alleviate PD progression. Additionally, the patients showed notable improvement in erectile function, with the IIEF-5 score significantly higher compared to before treatment. Furthermore, no patients experienced complications such as penile bruising, bleeding, or hematomas, confirming that LIPUS treatment for PD is safe.

Reference: Volume 2024, Article ID 7920485, 6 pages
In 2024, the internationally renowned andrology journal Andrologia published a clinical research paper titled “The Efficacy and Safety of Low-Intensity Pulsed Ultrasound Therapy for the Treatment of Peyronie’s Disease (A Report of 106 Cases): A Retrospective Study.” This study analyzed the treatment effects of 106 PD patients, with 56 patients in the LIPUS treatment group and 50 in the oral L-carnitine and Vitamin E treatment group (control group).
Research Background
Peyronie’s Disease (PD) is characterized by the formation of non-compliant fibrous plaques in the tunica albuginea of the penis, leading to pain and curvature during erection, thereby affecting erectile function. The global prevalence of PD ranges from 0.7% to 11%, and it increases with age. Most cases occur in men aged 50-60. Existing data shows that 40% of PD patients experience plaque enlargement over time, 47% remain stable, and only 13% recover spontaneously. Reports indicate that extracorporeal shock wave therapy (ESWT) can alleviate penile pain caused by PD, but its efficacy in reducing plaque size and penile curvature remains unclear. Unlike ESWT, LIPUS is a low-intensity pulsed ultrasound that has been shown to promote the regeneration of penile vascular, smooth muscle, and nerve tissues, reduce the release of local inflammatory factors, and thus repair and improve erectile function.
Study Objective
The aim of this study is to assess the efficacy and safety of low-intensity pulsed ultrasound (LIPUS) therapy for the treatment of Peyronie’s Disease (PD).
Product Features
WBL LIPUS is a low-intensity pulsed ultrasound therapy device. Its advantages include:Pulsed Design: The low-intensity pulsed ultrasound (LIPUS) significantly reduces the thermal effects associated with continuous ultrasound, optimizing mechanical effects.Non-invasive Treatment: No invasive procedures are required, reducing patient discomfort and risk.
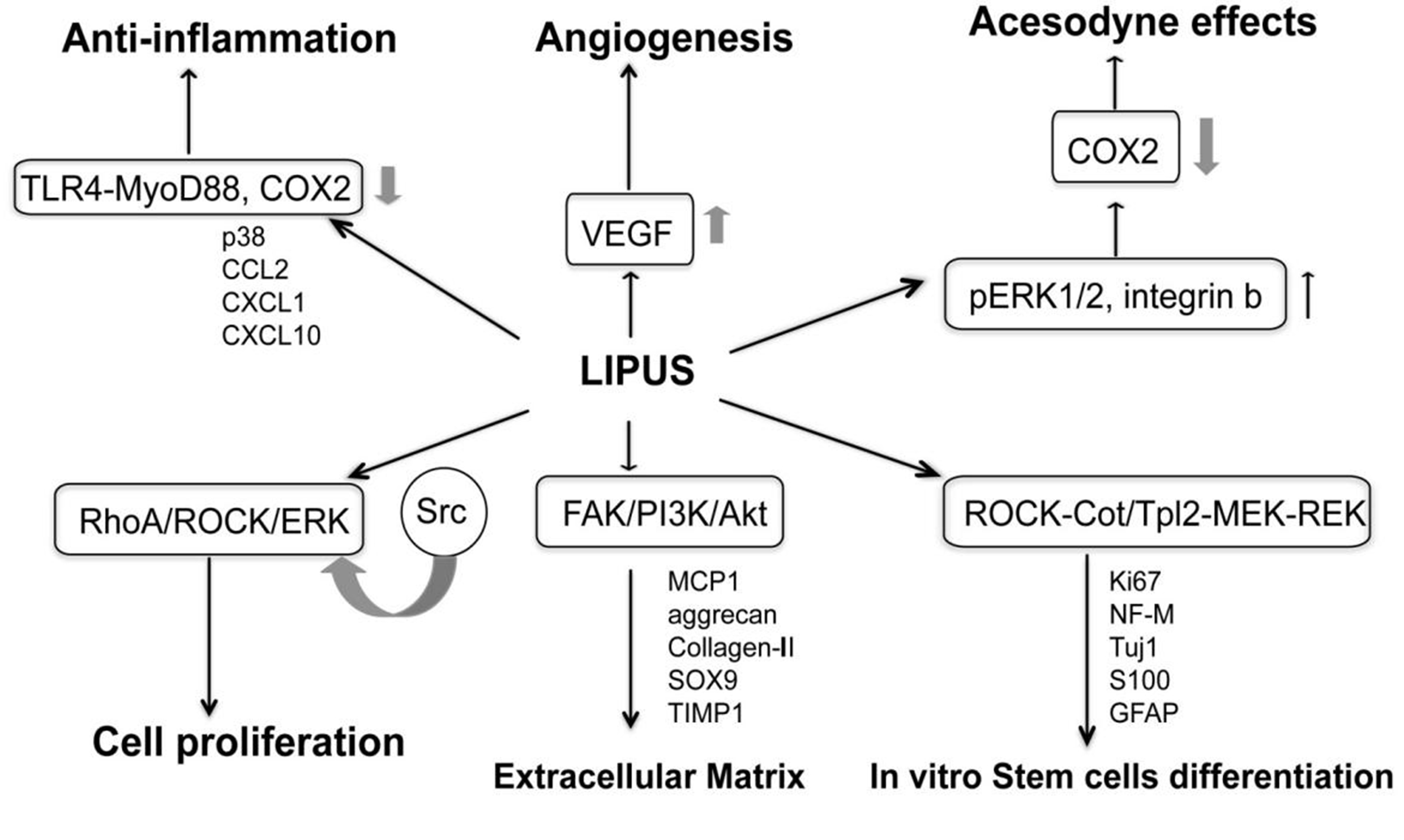
Biological Mechanism
LIPUS converts mechanical stimulation signals inside cells into biological signals, activating endogenous stem cells to repair endothelial cell function. It promotes angiogenesis and nerve regeneration, repairs the ratio of smooth muscle/collagen fibers in the penile cavernous tissue, and improves pathological changes in the tunica albuginea.
Study Subjects
(a) Erectile pain and penile curvature;
(b) Disease duration less than 12 months;
(c) Aged 18-70 years;
(d) No prior PD treatment;
(e) Only receiving medication or low-intensity pulsed ultrasound (LIPUS) treatment during follow-up.
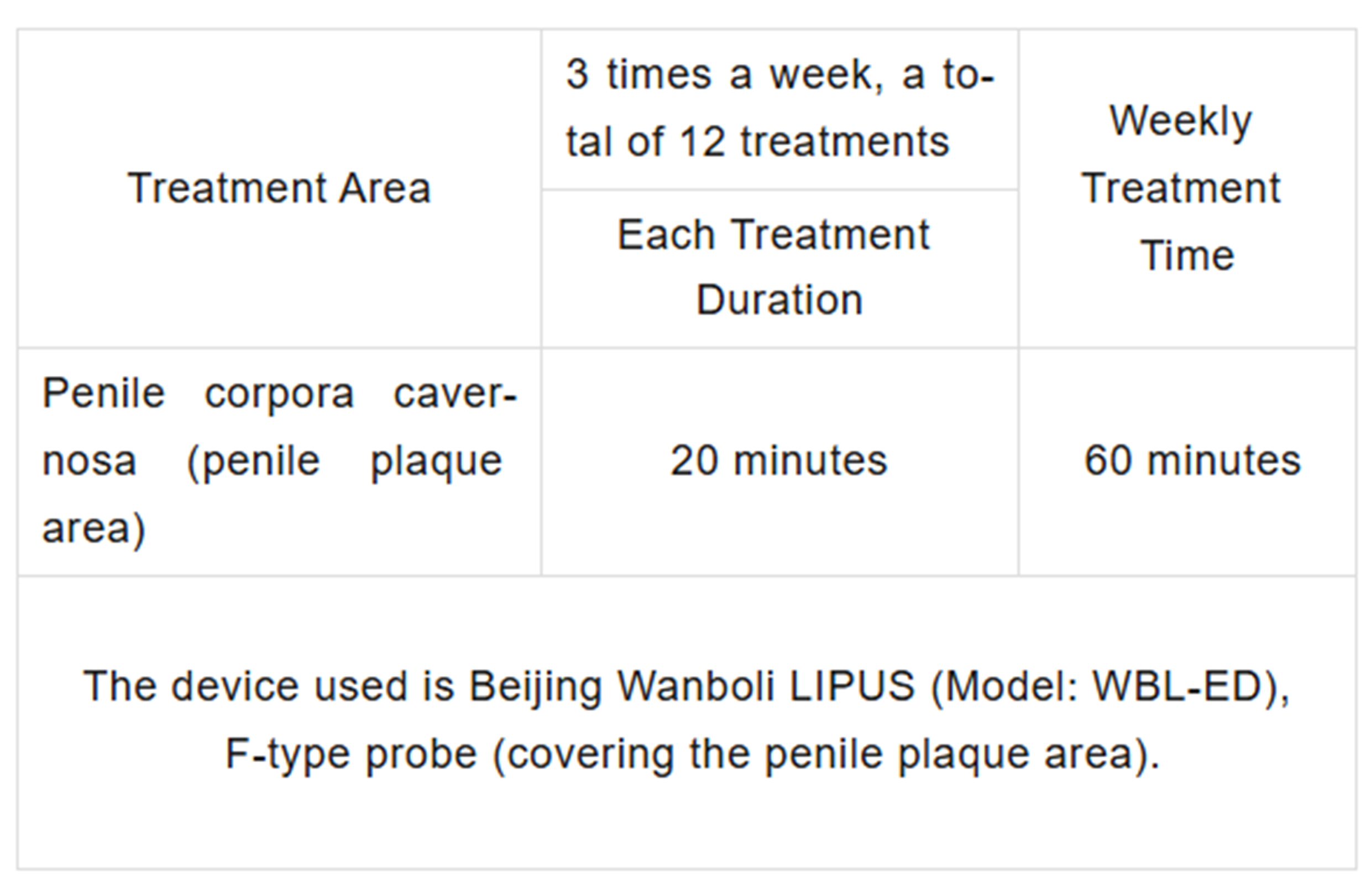
Treatment Method
Treatment Site, Mode of Contact, and Duration:
Patients receive ultrasound therapy 3 times per week for 4 consecutive weeks, totaling 12 treatments.
The treatment time and site are shown in the table below:
Observation Indicators
Pain during penile erection, erectile function, plaque size, and penile curvature were assessed in all patients before treatment, after 4 weeks of treatment, and at the 24-week follow-up to evaluate the effectiveness. Erectile pain was evaluated using the Visual Analog Scale (VAS), erectile function was assessed using the International Index of Erectile Function (IIEF-5), and plaque size was measured by ultrasound. After intracavernous injection of alprostadil to induce a full erection, the maximal penile curvature was measured using a goniometer.
Study Results
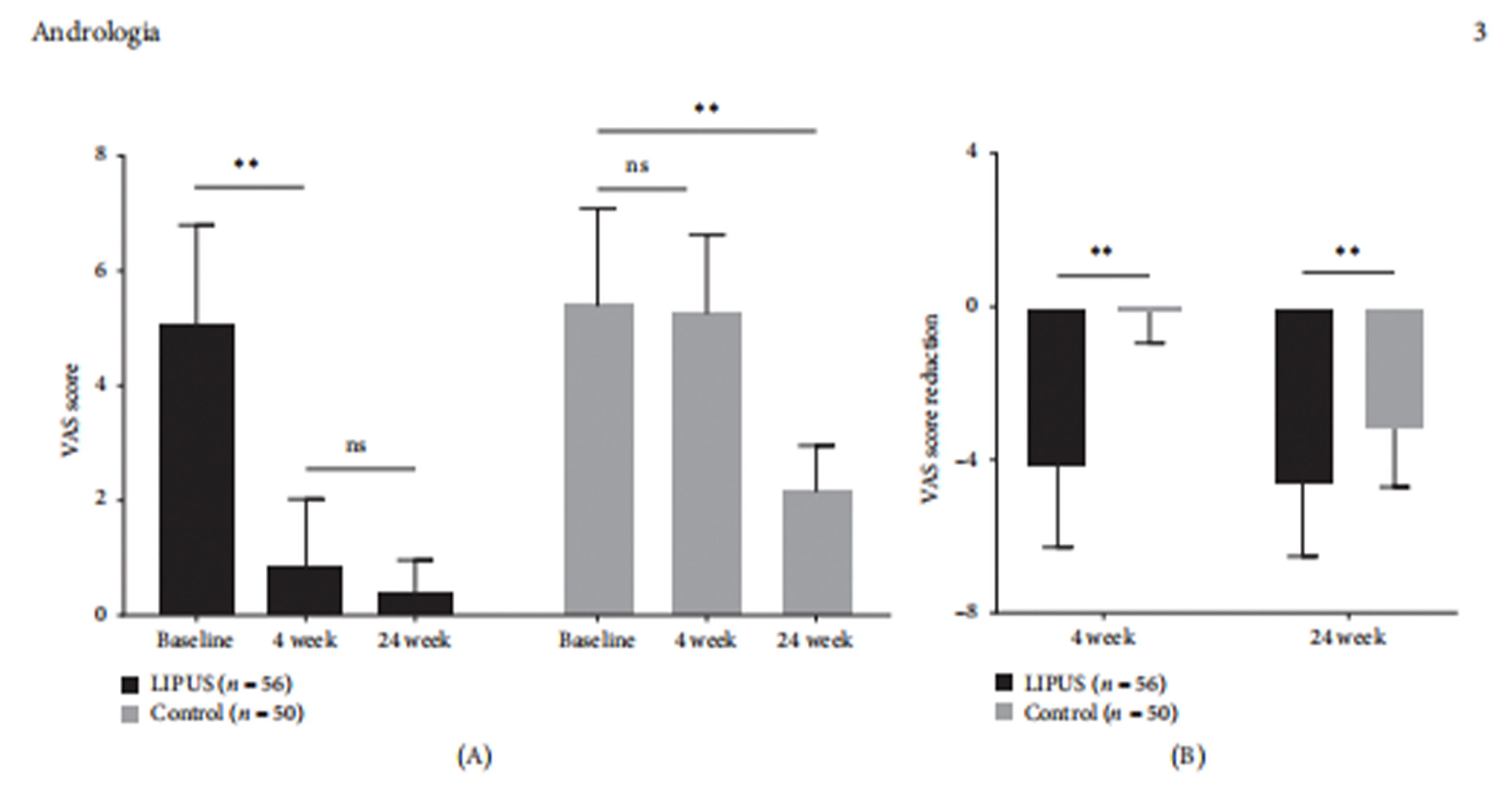
4-week VAS (Pain Score) Follow-up: The VAS score in the LIPUS group significantly decreased compared to baseline, while the control group showed no significant change.
24-week VAS (Pain Score) Follow-up: The VAS score in the control group decreased significantly but remained significantly higher than in the LIPUS group.
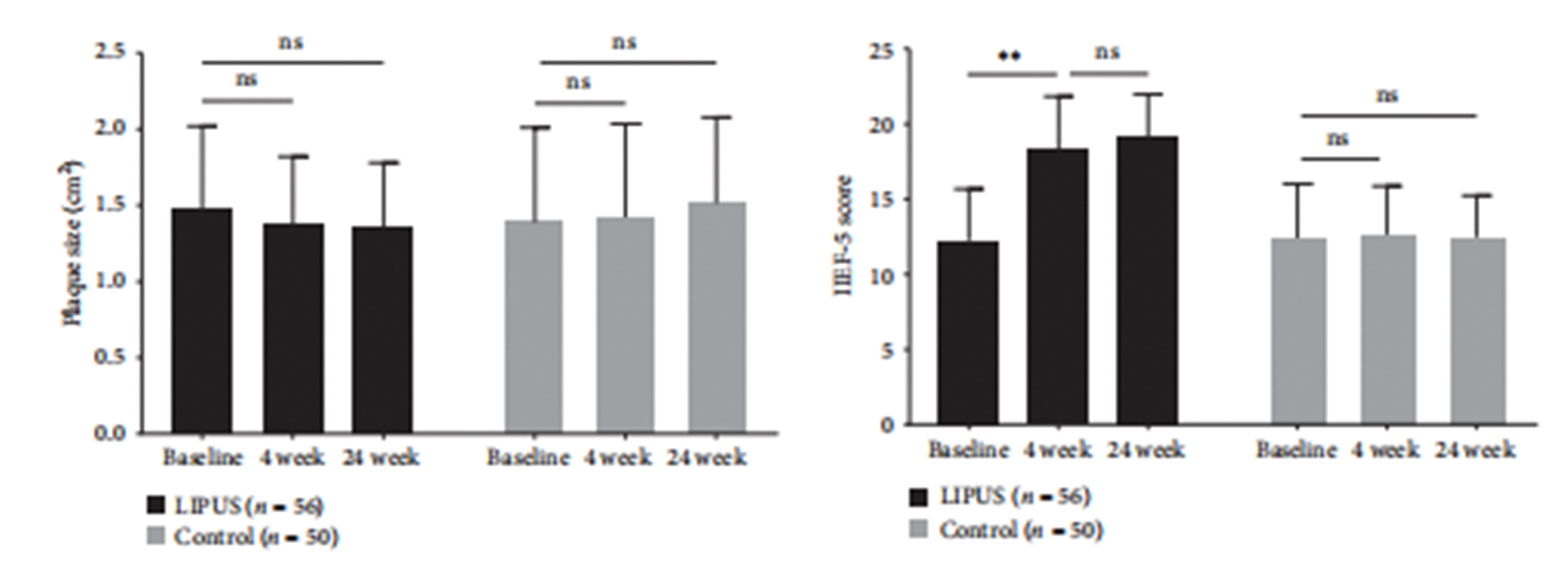
Erectile Function: The IIEF-5 score in the LIPUS group significantly improved compared to baseline, while the control group showed no significant changes at both the 4-week and 24-week follow-ups.
Plaque Size: The LIPUS group showed a trend toward plaque reduction, with the plaque boundary becoming significantly blurred. However, there were no significant differences between the two groups in terms of plaque size at the 4-week and 24-week follow-ups.
Penile Curvature: The LIPUS group showed significant improvement in penile curvature at the 4-week follow-up, while the control group showed no significant changes at either follow-up.
Safety: No complications such as penile pain, hematoma, or bruising occurred during or after LIPUS treatment.
Research Conclusion
In patients with Peyronie’s Disease (PD), LIPUS therapy significantly reduced erectile pain, improved erectile function, and decreased penile curvature, although the reduction in curvature was not statistically significant. The study also confirmed that LIPUS treatment is safe, with no significant side effects, and it is recommended for clinical use in the treatment of PD.
The LIPUS device used in this study was the WBL LIPUS model WBL-ED.
WBL Medical Devices Co., Ltd. is a pioneer in LIPUS technology. With its self-developed WBL-ED device, it was the first to apply low-intensity pulsed ultrasound technology in erectile dysfunction treatment, offering a non-invasive, safe, and effective solution. Since receiving approval from the China Food and Drug Administration (CFDA) in 2018, the WBL LIPUS has been recognized as an innovative medical product in China, showcasing WBL’s technological strength in the industry.
