
Conclusion: Both the thrice-weekly and twice-weekly LIPUS treatment protocols are safe and effective methods for treating mild to moderate ED, improving erectile function, erectile hardness, erectile satisfaction, as well as confidence and sexual relationships. The thrice-weekly protocol achieves comparable efficacy to the twice-weekly protocol in a shorter period.

Reference: JSM. 2022 Oct 19(10):1536-1545.
In 2020, a multi-center clinical study titled "Different Frequencies of LIPUS Treatment for ED: A Multi-center, Open-Label, Randomized Controlled Trial" was published by seven top medical centers in China in the Sexual Medicine Journal. This study adopted a randomized, open-label, multi-center, non-inferiority design. The study used a network-based central randomization system with patients from different hospitals enrolled through competitive grouping. Patients were randomly assigned to either the experimental group or control group in a 1:1 ratio. Both groups received LIPUS treatment, with the experimental group receiving treatment 3 times a week for a total of 16 sessions, and the control group receiving treatment 2 times a week for 16 sessions.
Background of the Study
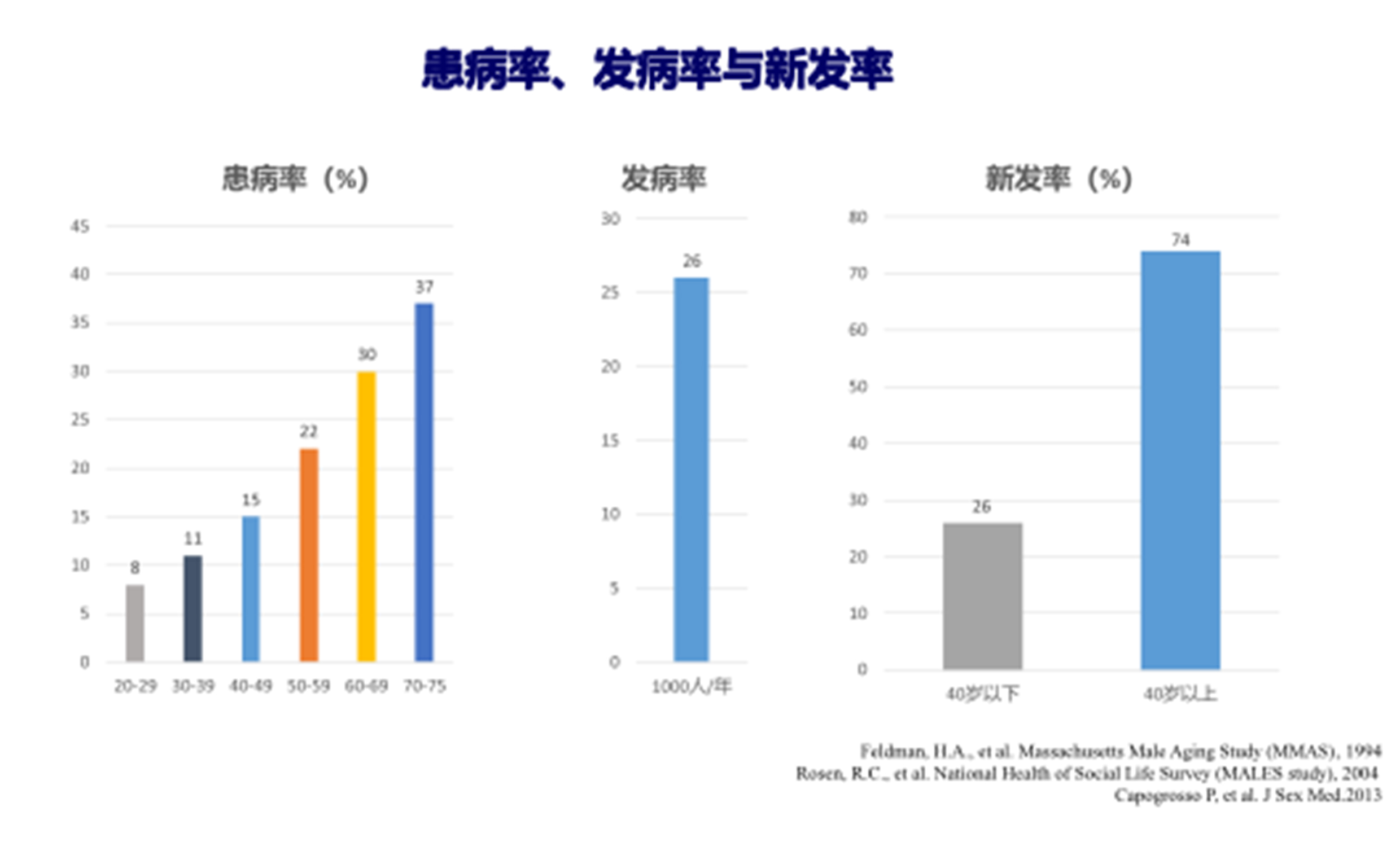
Erectile Dysfunction (ED) is a common condition among adult men, with a prevalence of about 52% in men aged 40–70 years and 22% in men under 40 years. It is estimated that by 2025, the global number of ED patients will exceed 300 million. ED severely affects the quality of life of patients and their partners and may also be an early symptom or risk signal of cardiovascular diseases. Phosphodiesterase type 5 (PDE5) inhibitors are the first-line treatment for ED, but the discontinuation rate due to adverse drug reactions ranges from 2% to 15%, and the rate of patients who do not respond to treatment is 22% to 37%. Second-line treatments, such as penile intracavernosal injections, pose risks of prolonged erections and penile fibrosis, leading to more than 90% of patients discontinuing treatment. Third-line treatments, such as penile prosthesis implantation, also carry unavoidable mechanical failures and infection risks, making it unacceptable for most patients. Recently, low-intensity pulsed ultrasound (LIPUS) and other micro-energy regenerative medical treatments have been explored for the clinical treatment of ED, showing some efficacy and safety.
Study Objective
This study aims to compare the efficacy and safety of two different LIPUS treatment frequencies for mild to moderate ED through a multi-center, open-label, randomized controlled trial. The study will explore the appropriate treatment frequency or treatment time cost while ensuring efficacy and safety.
Product Features
WBL LIPUS is a low-intensity pulsed ultrasound therapy with the following advantages:Pulse design: Low-intensity pulsed ultrasound (LIPUS) significantly reduces the thermal effects of continuous ultrasound treatment devices and optimizes the mechanical effects.Non-invasive treatment: No invasive procedures are required, reducing patient discomfort and risk.
Biological Mechanism
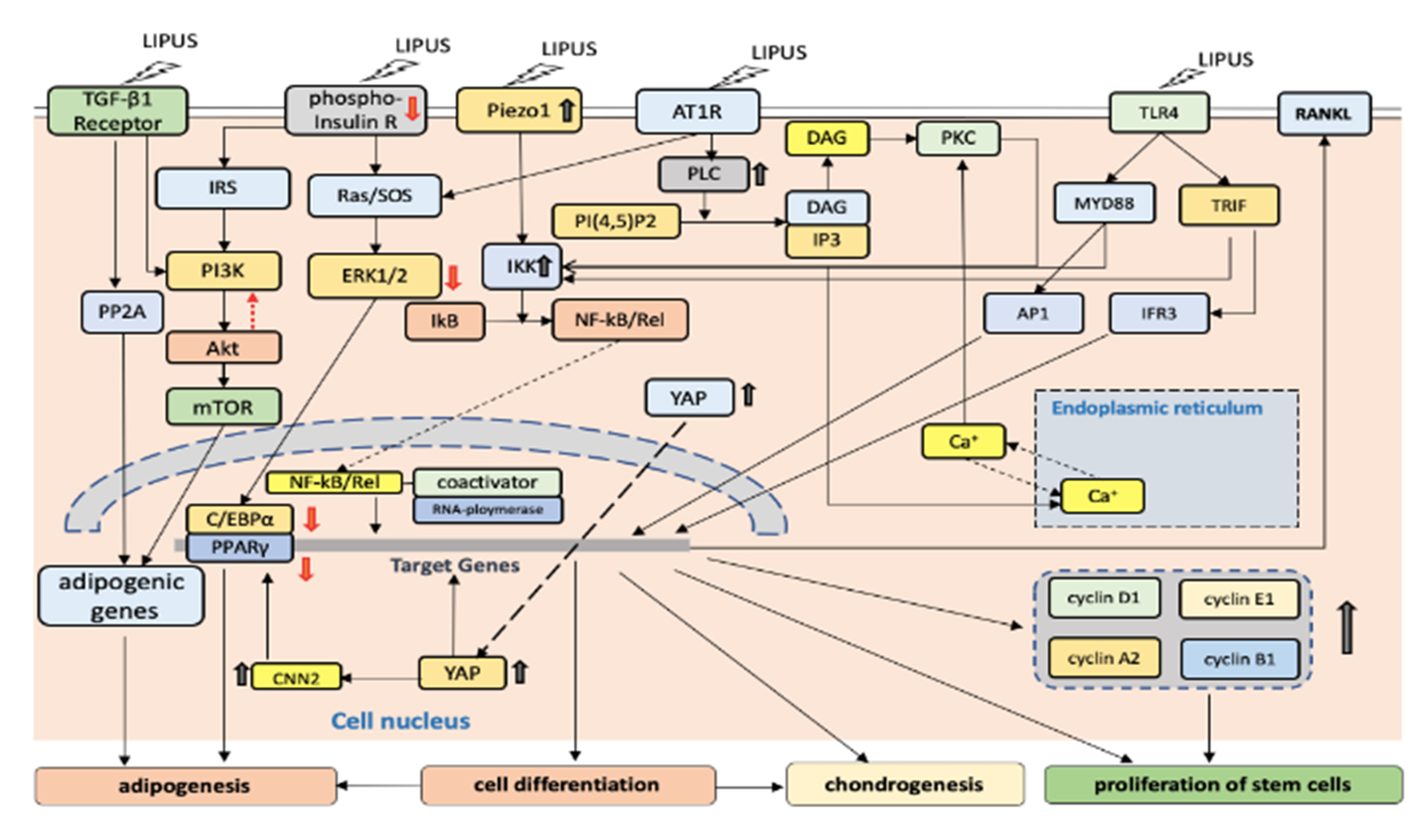
LIPUS is a promising new treatment for ED that has gained recent research attention. Numerous studies have shown that LIPUS can induce and recruit the proliferation and differentiation of stem cells through mechanical force signal transmission, including pluripotent stem cells derived from neural crest stem cells, adipose progenitor cells, and mesenchymal stem cells. Research has found that the penis contains endogenous penile stem/progenitor cells, and micro-energy ultrasound stimulation can induce these cells to differentiate into endothelial cells, smooth muscle cells, and neurons, providing a molecular basis for LIPUS treatment to repair pathological changes in ED cavernous tissue.

Study Subjects
1.Aged 20 to 60 years.
2.Overall healthy with a stable heterosexual partner for more than 3 months.
3.ED duration of at least 3 months but less than 10 years.
4.Mild to moderate ED, with an International Index of Erectile Function - Erectile Function (IIEF-EF) score between 11 and 25 points.
5.No use of PDE5 inhibitors or other ED treatments within 2 weeks before the grouping.
Treatment Methods
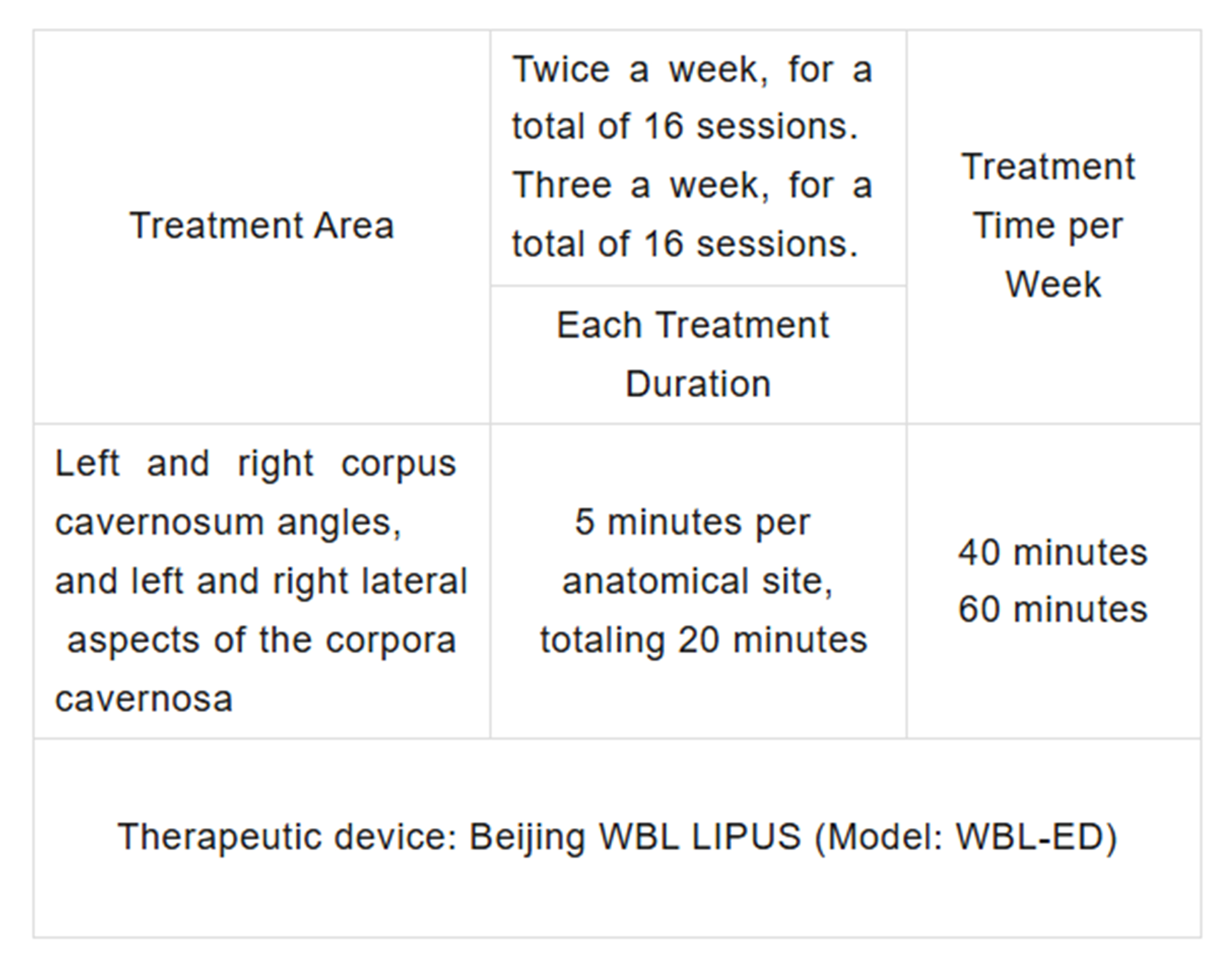
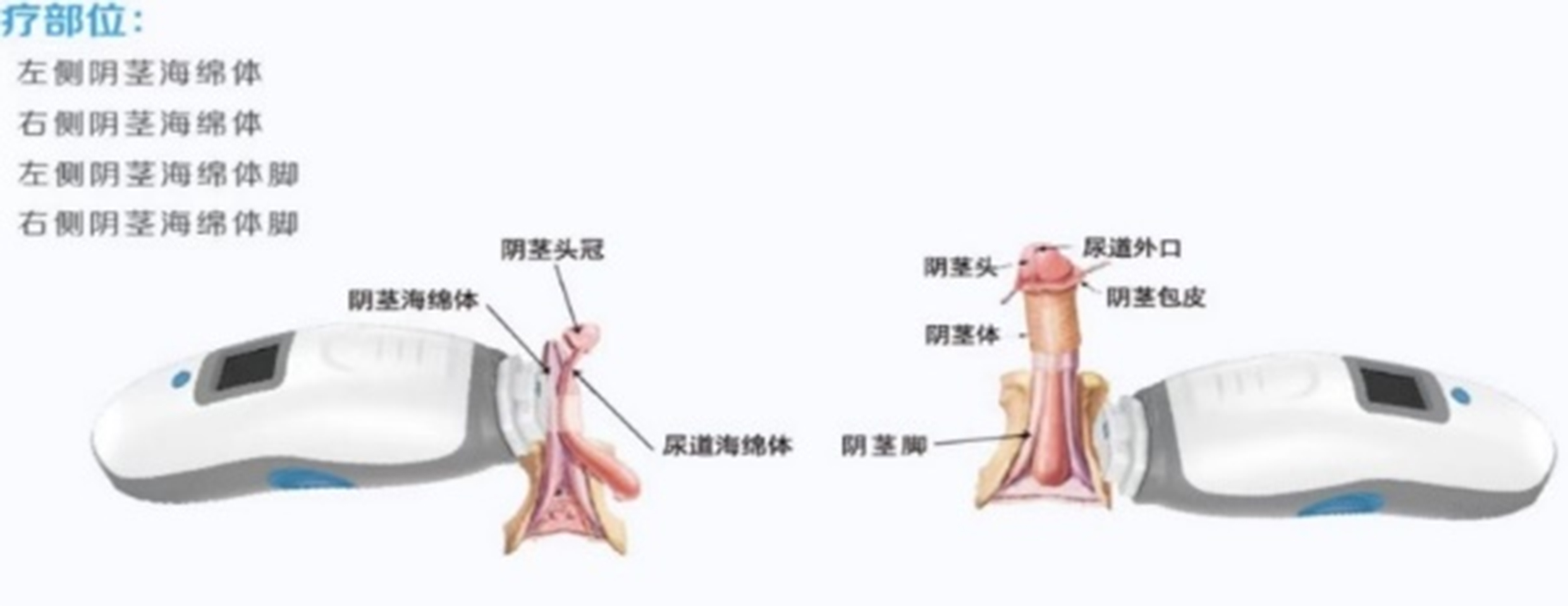
1、Treatment Site, Contact Method, and Duration:
Patients received LIPUS treatment twice per week or thrice per week for 16 sessions, with four sites treated in each session.
2、The treatment time and locations are as follows:
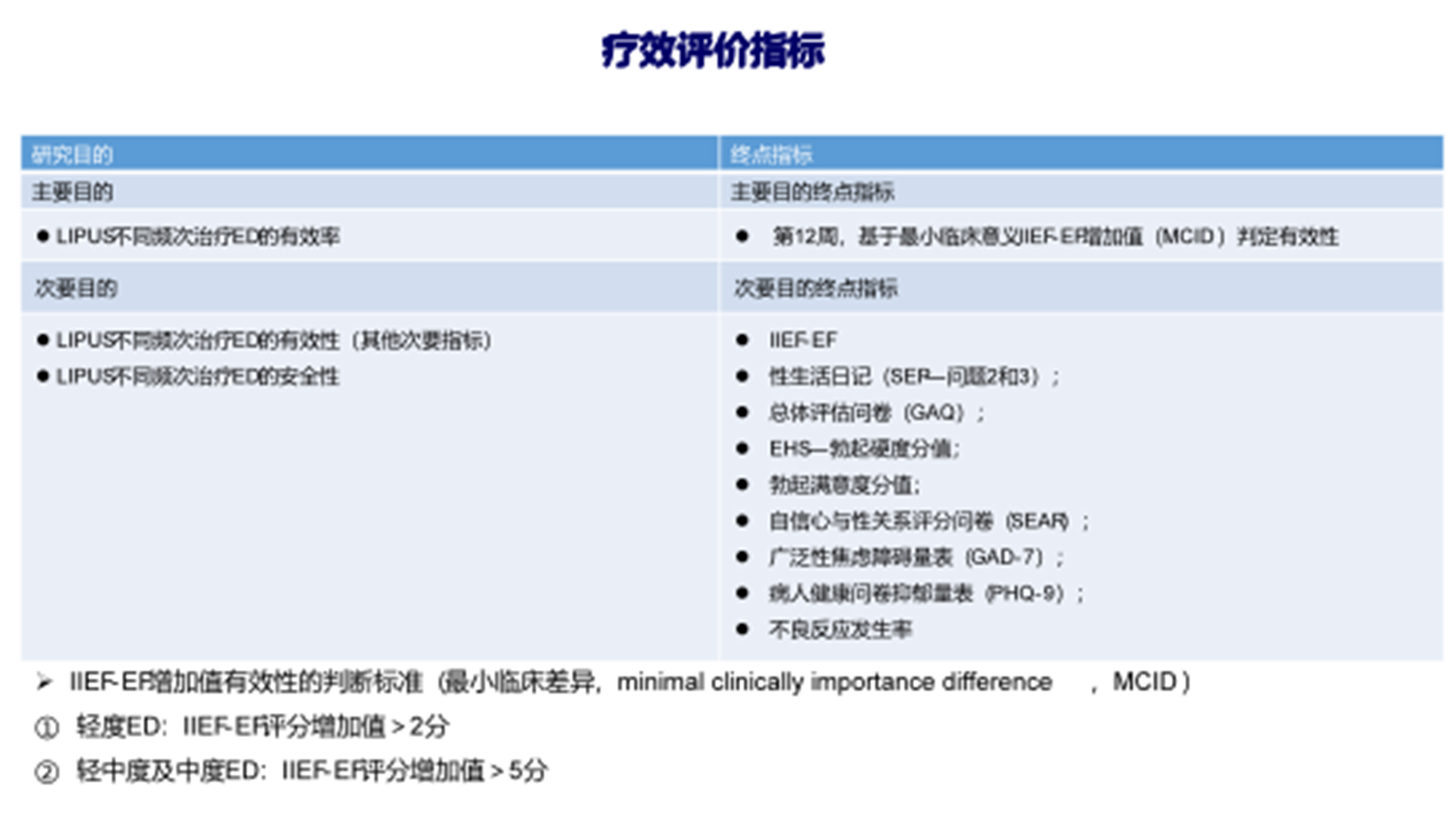
Observation Indicators
The primary efficacy indicator is IIEF-EF. The change in IIEF-EF from baseline was described at the end of the 8th treatment (Follow-up 1), 16th treatment (Follow-up 2), and the 12th week (final follow-up).
Study Results
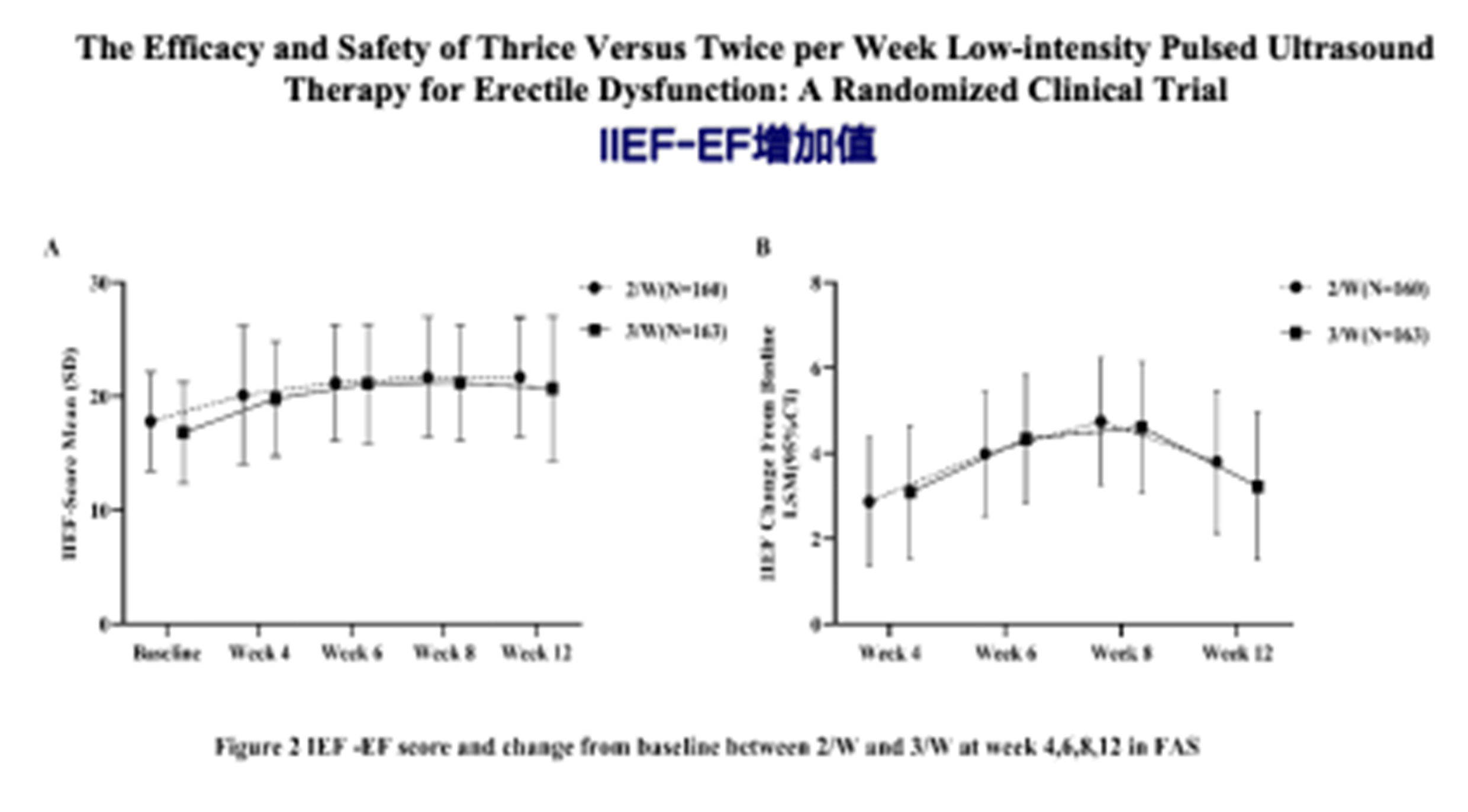
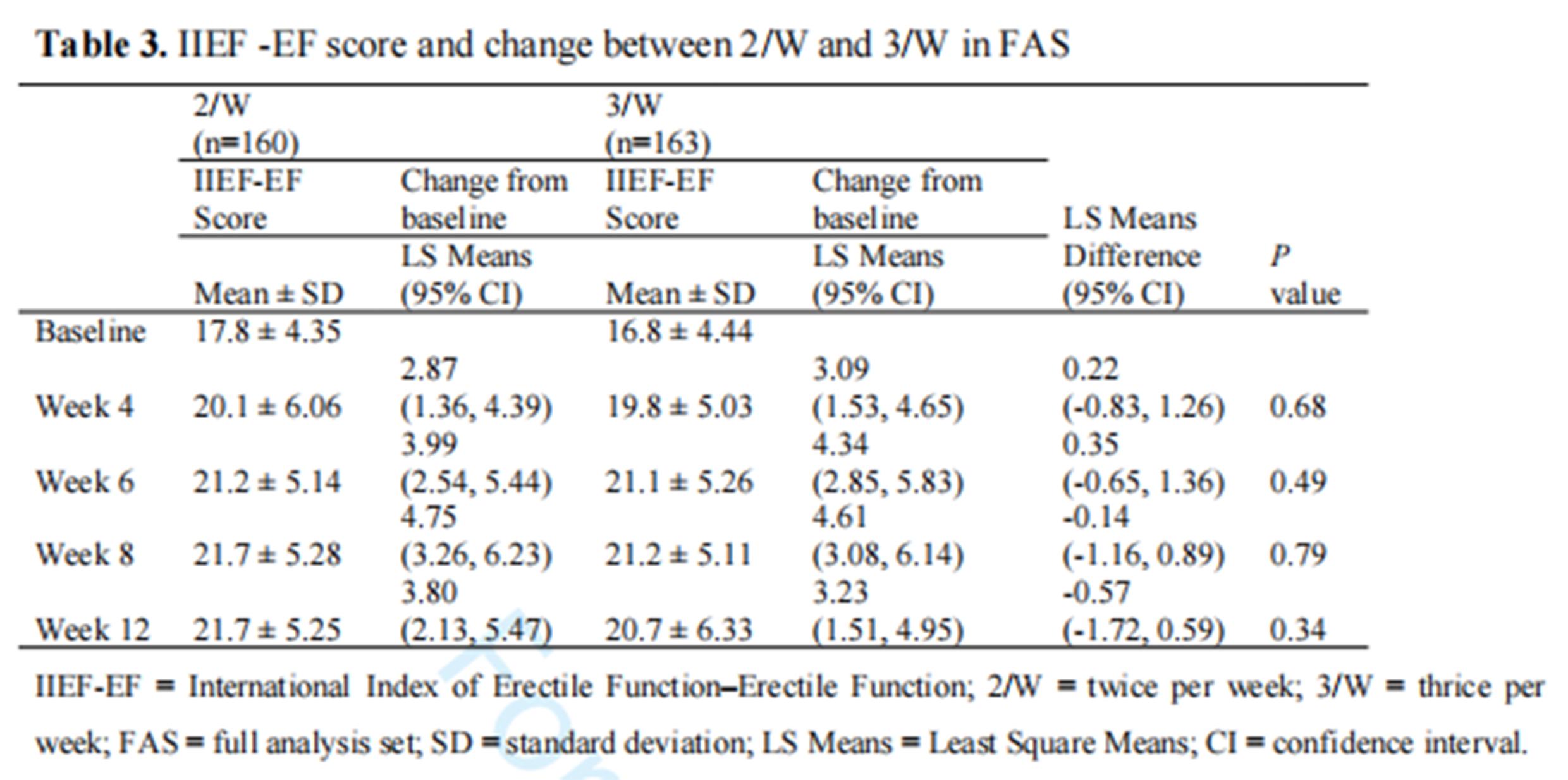
1. A total of 349 patients were screened for the study, with 323 enrolled and 279 completing the trial. The experimental group had 140 patients and the control group had 139 patients. At the endpoint of the follow-up, the IIEF-EF score increase at week 4 showed statistical differences between the groups. However, at weeks 8 and 12, no significant difference in efficacy was observed between the two groups after adjusting for baseline IIEF scores and center effects.
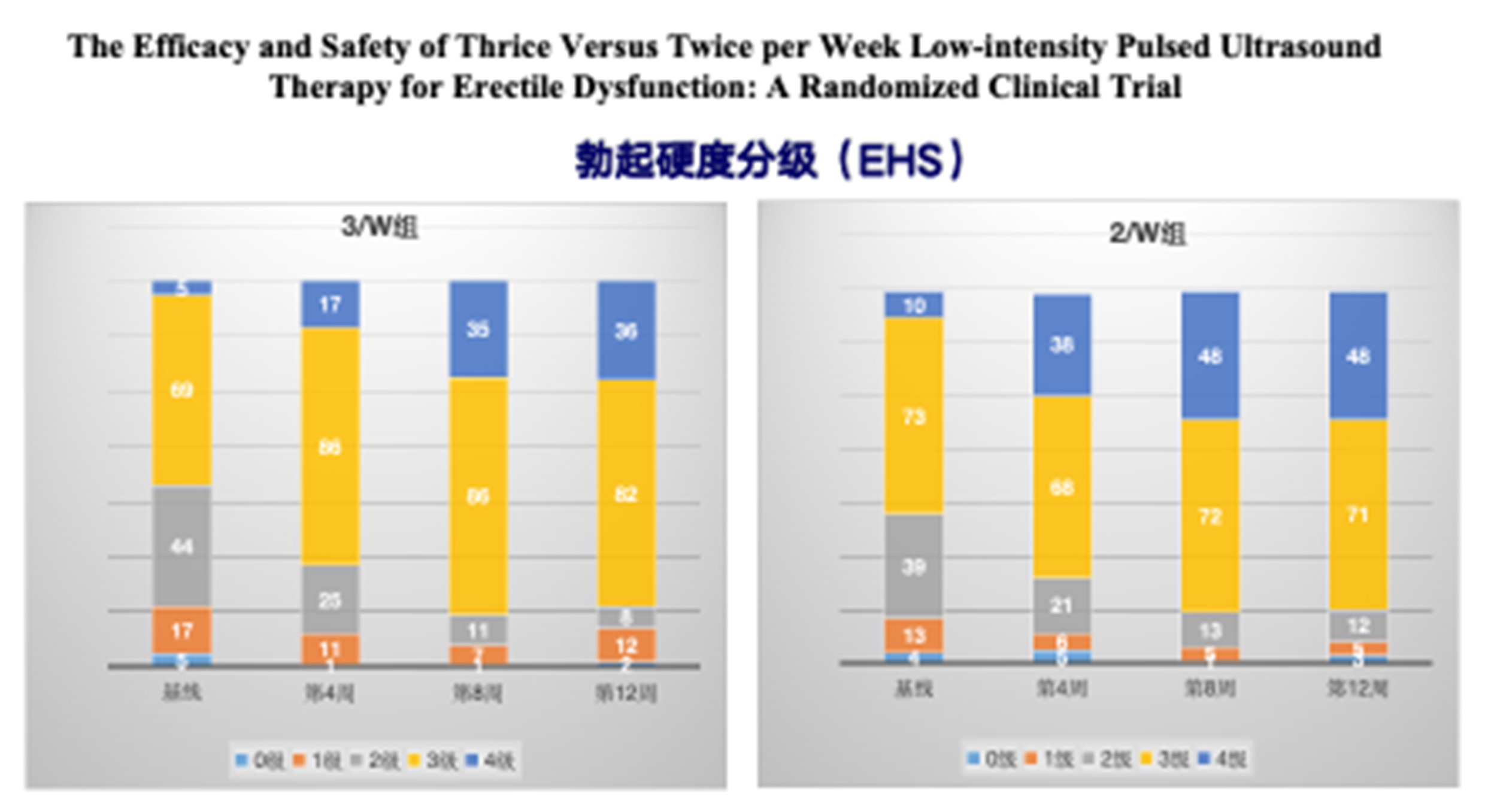
2. By week 12, the proportion of patients achieving an erectile hardness score (EHS) of 3/4 was 84% in the experimental group and 83.5% in the control group. A statistical difference was observed at week 4, but no differences were found at weeks 8 and.
3. There were no statistical differences in erectile hardness, erectile satisfaction, morning erections, stimulated erections, sexual intercourse completion rate, sexual relationship satisfaction, or other secondary outcomes between the two groups at the 8th, 16th, and final follow-up.
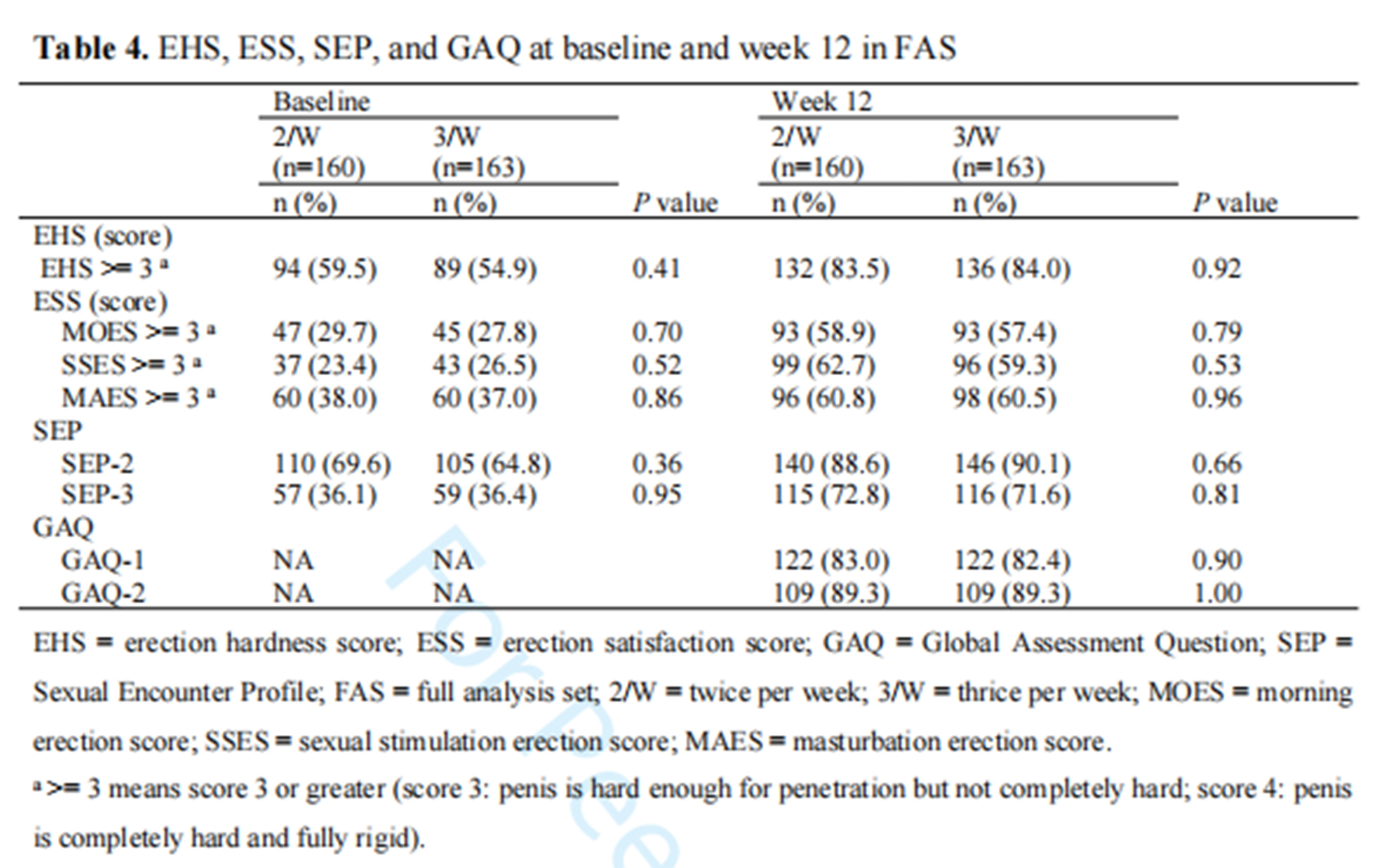
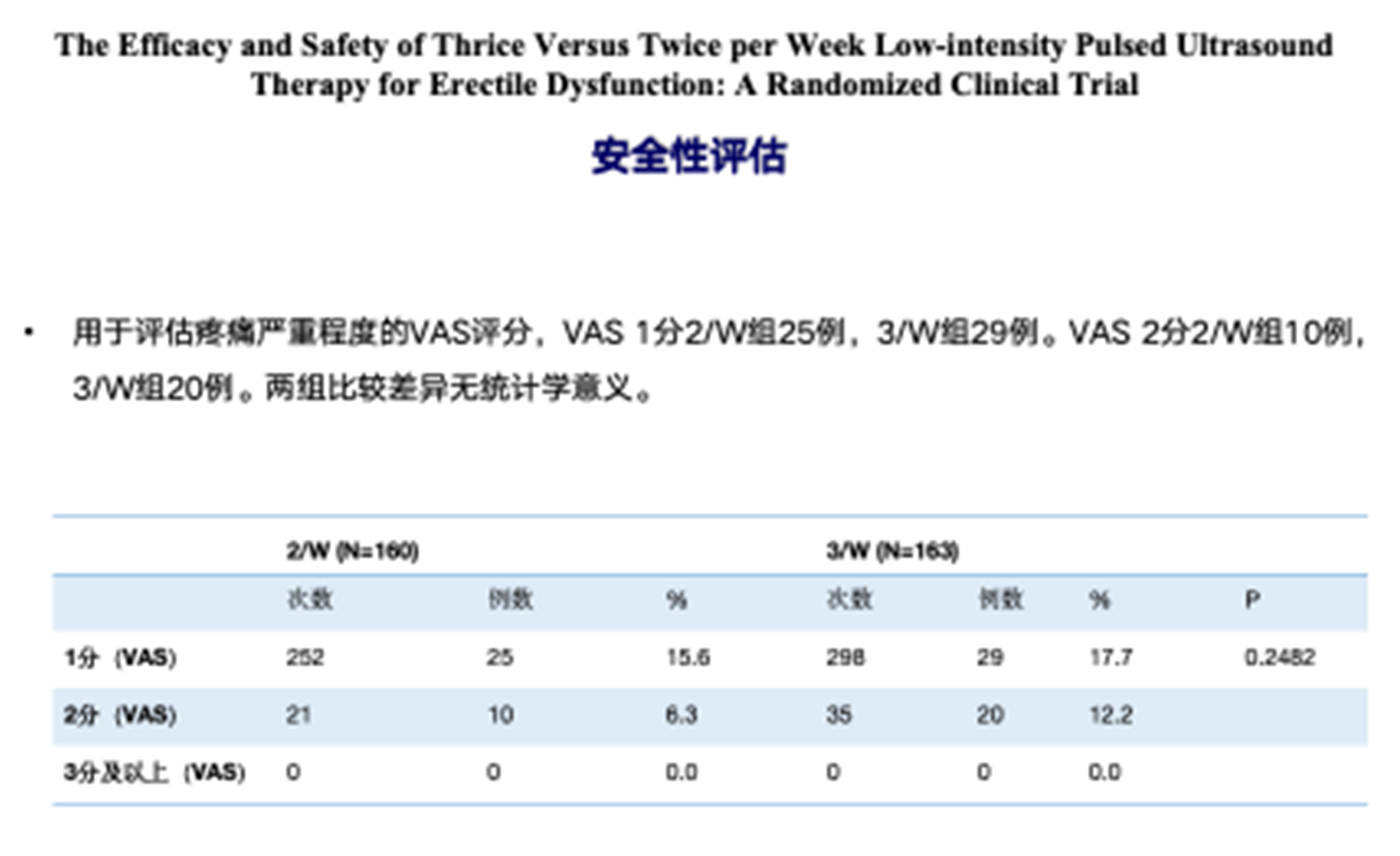
4.No significant adverse events were reported in either group during the study period. All adverse events were unrelated to the LIPUS device, with no severe adverse events or events related to the treatment observed. No bruising, hematoma, or hematuria was reported.
Conclusion
Both the thrice-weekly and twice-weekly LIPUS treatment protocols are safe and effective methods for treating mild to moderate ED, improving erectile function, erectile hardness, erectile satisfaction, as well as confidence and sexual relationships. The thrice-weekly protocol achieves comparable efficacy to the twice-weekly protocol in a shorter period. The difference in efficacy between the two treatment frequency protocols is not influenced by the severity of ED, age, or the presence of underlying diseases.
The LIPUS device used in this study is the WBL-ED model by WBL Medical.
WBL Medical Instruments Co., Ltd. is a pioneer in LIPUS technology. With its self-developed LIPUS-ED device, it was the first to apply low-intensity pulsed ultrasound technology for the treatment of erectile dysfunction, providing a non-invasive, safe, and efficient new solution. Since its approval by the China Food and Drug Administration (CFDA) in 2018, WBL LIPUS has not only filled a domestic and international technical gap in this field but also won the China National Medical Device Innovation Product Award, fully demonstrating WBL's leading position and technical strength in the industry.
