
Conclusion: The results of this clinical trial suggest that the WBL-ED low-energy pulsed ultrasound device, produced by Beijing WBL Medical Device Co., Ltd., is effective in treating erectile dysfunction (ED). It demonstrates safety and efficacy for the clinical treatment of ED.
Reference: Transl Androl Urol 2019 | http://dx.doi.org/10.21037/tau.2019.07.03
Beijing WBL Medical Device Co., Ltd. collaborated with the Department of Andrology at Peking University First Hospital's Molecular Biology Laboratory and the Knuppe Molecular Urology Laboratory at the University of California, San Francisco, to develop the novel LIPUS device for ED treatment. In 2019, five major domestic medical centers jointly completed the clinical study titled "WBL-ED Low-Energy Pulsed Ultrasound Device for Erectile Dysfunction Treatment" and published it in the Original Article. This research, combining safety and efficacy analysis, confirmed that the low-energy pulsed ultrasound device is effective in improving erectile function, providing a safe and effective treatment for ED.
Background
Erectile function requires the coordinated effort of multiple organ systems, including psychological, endocrine, vascular, and neural regulation. Erectile Dysfunction (ED) is a common condition, affecting more than 50% of men between the ages of 40 and 70 (1). A 2010 report from the European Male Aging Study (EMAS) highlighted that a third of men in their population (prevalence of 30-64%) were dissatisfied with their overall erectile function or sexual relationships. Previous studies on LIPUS therapy have shown improvements in pathological changes in penile erectile tissue, increased erectile function (penile corpus cavernosum pressure, ICP) in streptozotocin (STZ)-induced diabetic rats, and enhanced vascular endothelial and smooth muscle content. LIPUS also increased the expression of eNOS and nNOS, reduced collagen and fibrosis, and downregulated the TGF-β1/Smad/CTGF signaling pathway. No treatment-related adverse events (AEs) were observed in animal studies. To explore the clinical efficacy and safety of LIPUS in ED patients, a multi-center, randomized, double-blind, placebo-controlled clinical trial was conducted.
Objective
This study was designed as a multi-center, randomized, double-blind, placebo-controlled trial, with 120 mild to moderate ED patients treated at five medical centers. The study aimed to assess the clinical efficacy and safety of the ultrasound therapy device for ED.
Product Features
WBL LIPUS is a low-intensity pulsed ultrasound therapy, characterized by its pulsed design: the low-intensity pulsed ultrasound (LIPUS) significantly reduces the thermal effects of continuous ultrasound devices, optimizing the mechanical effects. It is a non-invasive treatment, eliminating the need for invasive procedures and reducing patient discomfort and risks.
Biological Mechanism
LIPUS converts mechanical stimulation signals within cells into biological signals by activating endogenous stem cells to repair vascular endothelial cell function. It promotes neovascularization and nerve regeneration, repairs the balance of smooth muscle/collagen fibers in the penile corpus cavernosum, and corrects pathological changes in the corpus cavernosum white membrane.
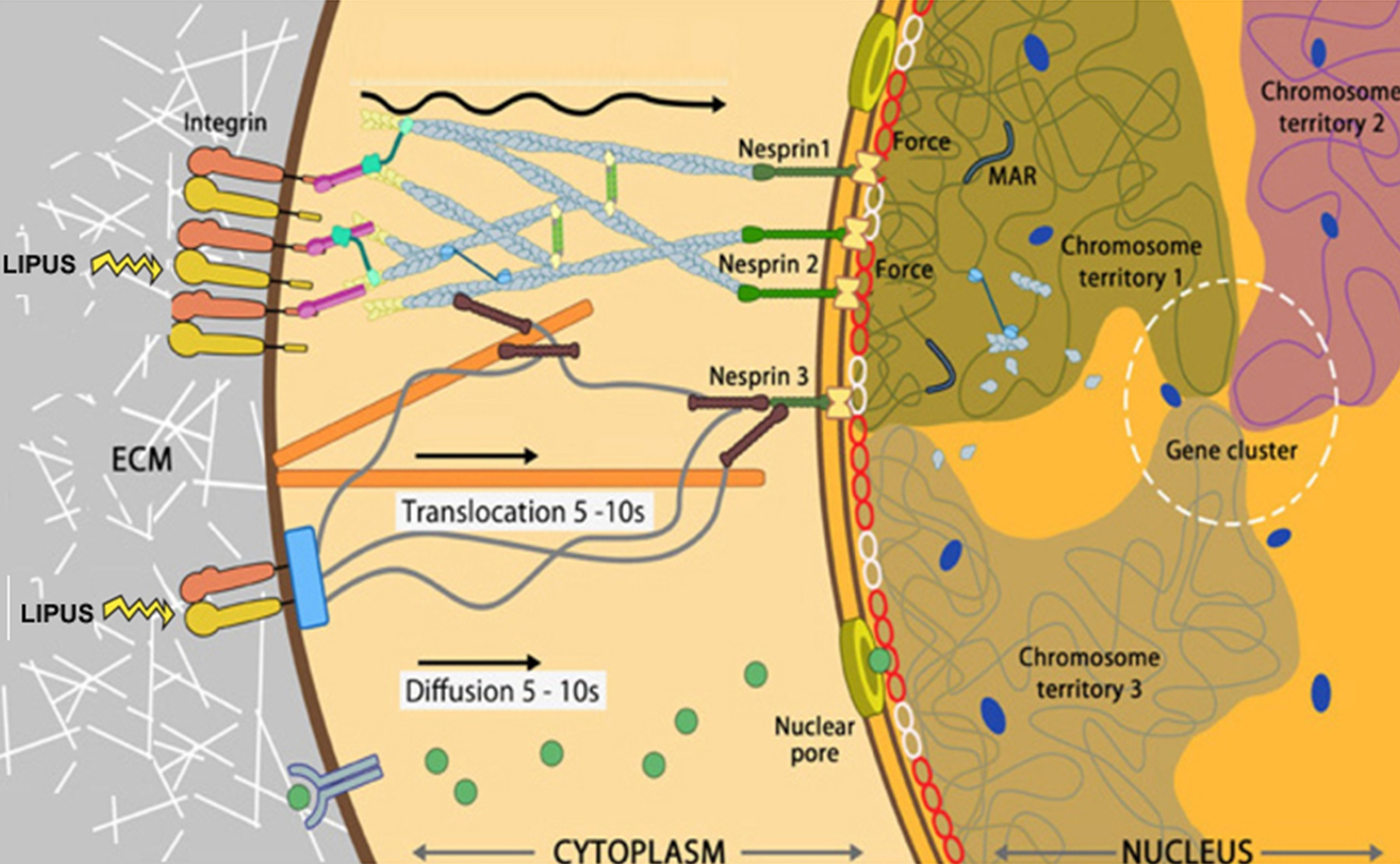
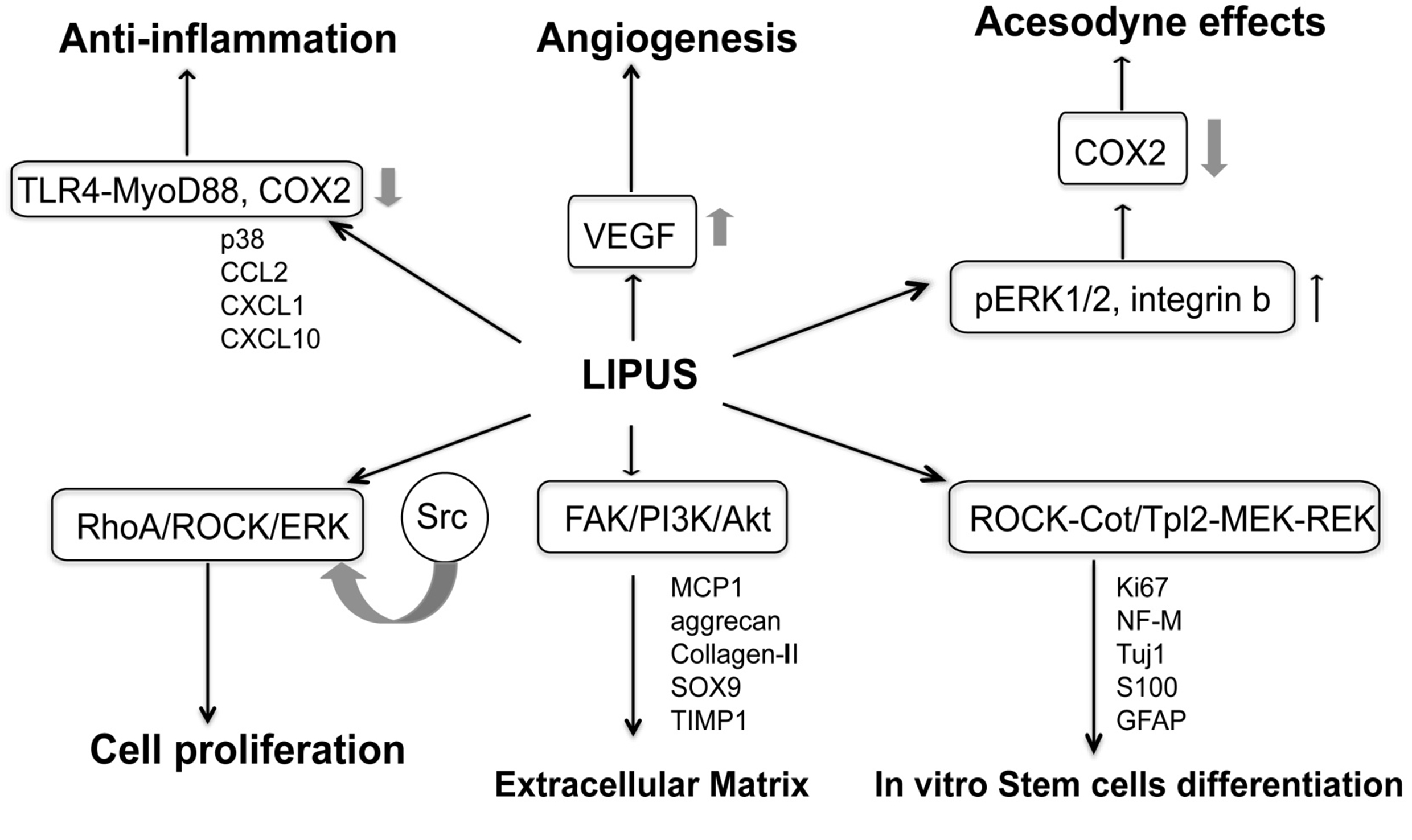
(Guiting Lin, Tom F Lue, et al, 2016)
Study Participants
Men aged 20-60 years;
mild to moderate ED (International Index of Erectile Function (IIEF-5) scores between 8 and 21);
with a history of ED for at least 3 months.
Treatment Method
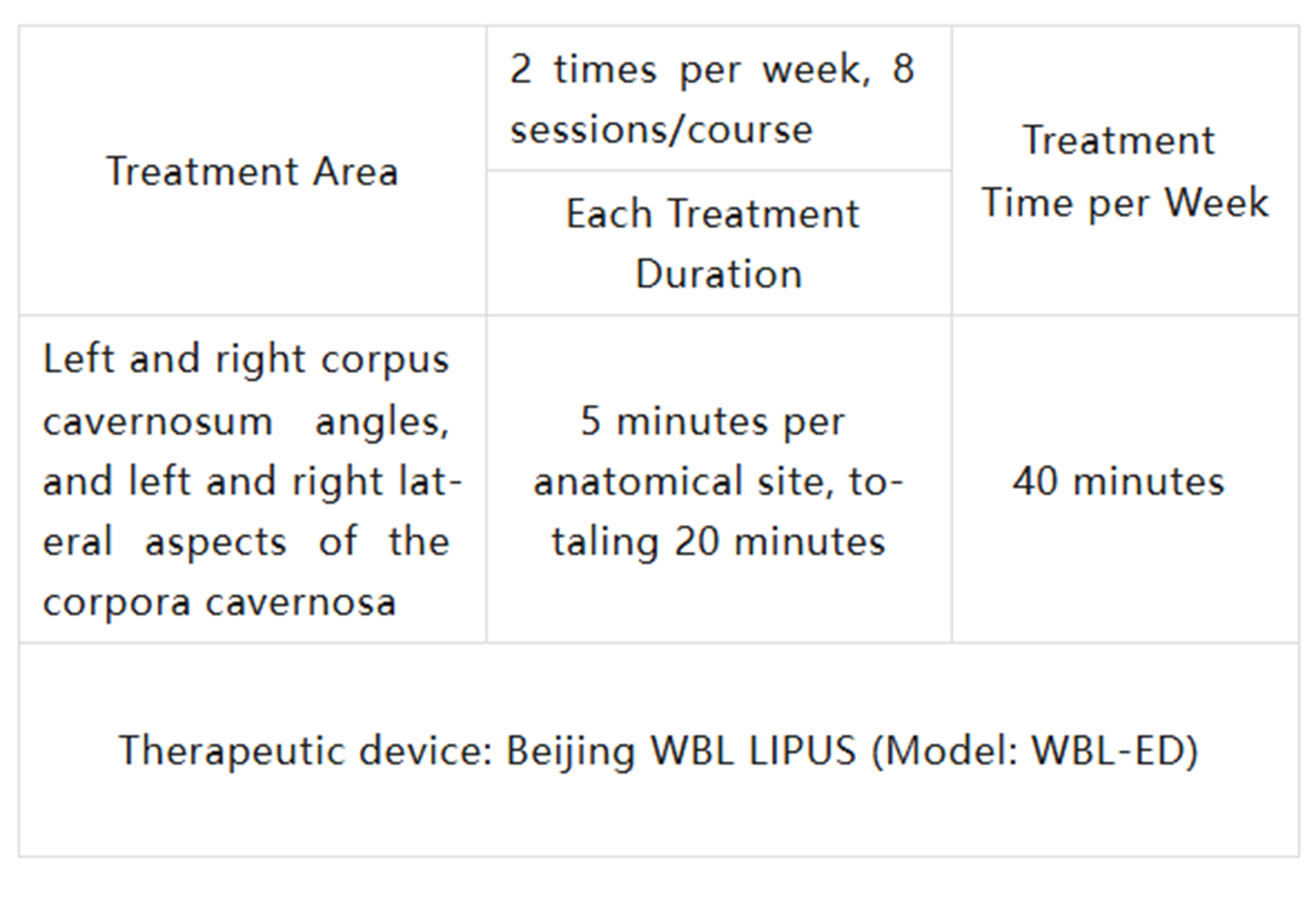
Patients were treated with the ultrasound device twice a week for 4 consecutive weeks (8 sessions per treatment course), with treatment applied to 4 different areas of the body.
The treatment time and areas are listed in the table below:
Observation Indicators
The primary indicator was the International Index of Erectile Function (IIEF-5) score. Secondary indicators included the Sexual Encounter Profile (SEP) problems 2 and 3, the Global Assessment Questionnaire (GAQ), Erection Hardness Score (EHS), and the Erectile Satisfaction Score.
Study Results
The trial results showed that the ultrasound therapy device significantly outperformed the control group in terms of the primary efficacy endpoint: the 12-week IIEF-EF score increase and effectiveness rate. The 12-week evaluation results showed 71.01% for the treatment group and 17.65% for the control group. These results demonstrate that the IIEF-EF score change and effectiveness rate at 12 weeks were significantly better in the treatment group. Secondary efficacy measures, including SEP (problems 2 and 3), GAQ, EHS, and erectile satisfaction scores, also showed superior outcomes in the treatment group compared to the control group.
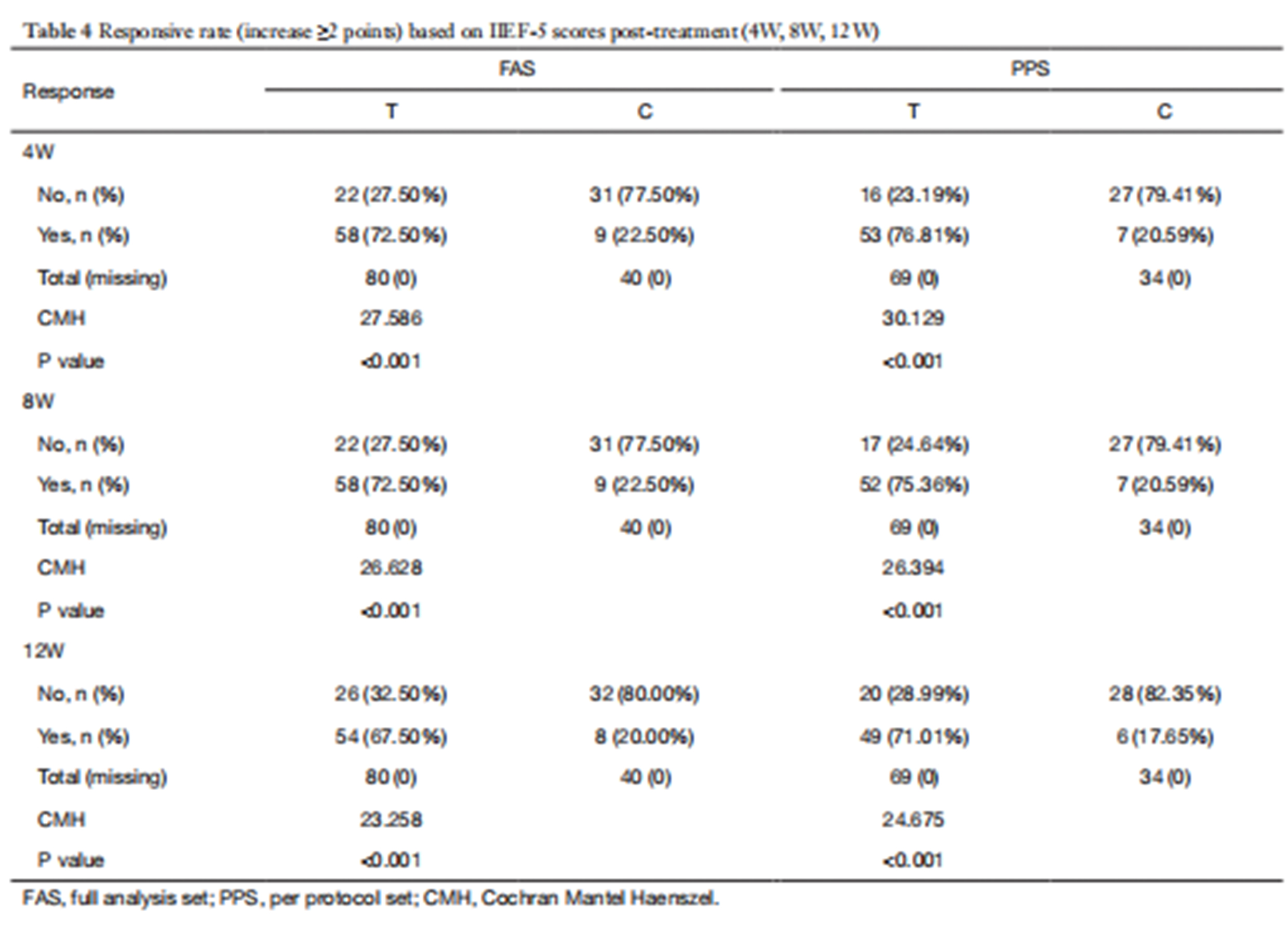
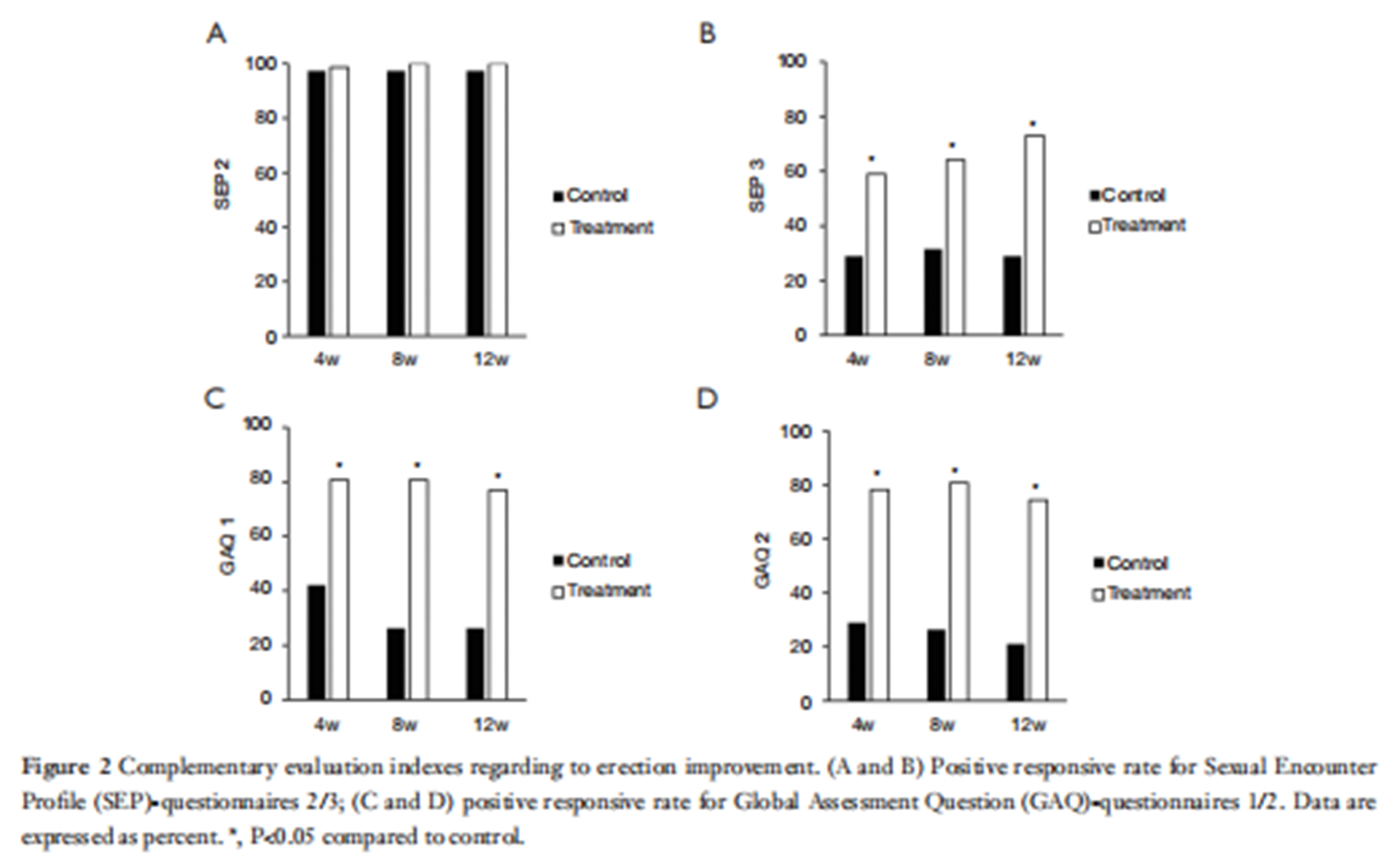
Study Conclusion
These results suggest that LIPUS is an effective treatment for mild to moderate ED without significant adverse events related to the mechanical forces of LIPUS. The mechanism of action is linked to the non-invasive mechanical stimulation of low-energy pulsed ultrasound, which transduces mechanical biological signals to regulate tissue cellular effects, including the modulation of endogenous stem cells. This leads to the repair of pathological changes in the penile corpus cavernosum, including repair of vascular endothelial cell damage, nerve damage, and fibrosis.
The device used in this trial was the WBL LIPUS model WBL-ED.
WBL Medical Device Co., Ltd. is a pioneer in LIPUS technology. With its self-developed WBL-ED device, it was the first to apply low-intensity pulsed ultrasound technology for the treatment of erectile dysfunction. Since receiving approval from the China Food and Drug Administration (CFDA) in 2018, WBL LIPUS has filled a technological gap both domestically and internationally, earning the title of National Medical Device Innovation Product in China. This achievement underscores WBL’s leading position and technological strength in the industry.
