
Conclusion: The study results show that after treatment with WBL low-intensity pulsed ultrasound (LIPUS) for mild, moderate, and severe erectile dysfunction (ED), various evaluation indicators at the 4-week and 12-week follow-ups were significantly improved compared to pre-treatment levels. The SEP (questions 2 and 3) and GAQ questionnaires had higher scores at both the 4-week and 12-week follow-ups compared to pre-treatment; penile color Doppler ultrasound (CDDU) showed that PSV levels at the 12-week follow-up were higher than pre-treatment levels, and EDV levels at the 12-week follow-up were lower than pre-treatment levels. No adverse reactions such as local bleeding, purpura, pain, numbness, or tingling occurred during treatment and follow-up.

Reference: JModUrol, Vol.28 No.11 Nov.2023
In 2023, an article titled "Evaluation of the Efficacy of Low-Intensity Pulsed Ultrasound for Treating Erectile Dysfunction" was published in the Modern Urology Journal, Vol. 28, No. 11, November 2023. The article retrospectively analyzed ED patients who visited between June 2020 and June 2022. Patients were divided into mild (17-25 points), moderate (11-16 points), and severe (0-10 points) subgroups based on the IIEF-EF scores. The study analyzed the total effective rate, erection hardness rating (EHS), Sexual Encounter Profile (SEP) questions, General Assessment Questionnaire (GAQ), peak systolic velocity (PSV), end-diastolic velocity (EDV), and adverse reactions at baseline, 4-week, and 12-week follow-ups for each group.
Background
Erectile dysfunction (ED) can severely impact a patient's relationship with their partner and lead to psychological issues such as depression and anxiety. Research shows that the prevalence of ED in Asia can range from 22.4% to 69.2%. Although non-surgical treatments such as medications and devices have been proven to be safe and effective, they do not reverse the penile vascular changes caused by subtle blood pressure variations. Low-intensity pulsed ultrasound (LIPUS) is an emerging non-invasive treatment that has shown significant efficacy in various fields, including nerve injury repair and rehabilitation. While studies suggest LIPUS has clinical value in improving erectile function, its clinical application in ED patients remains under-researched, and its safety and efficacy need further validation. Therefore, we investigated the efficacy and safety of LIPUS in ED patients.
Objective
To explore the efficacy of low-intensity pulsed ultrasound (LIPUS) in treating erectile dysfunction (ED) in men.
Product Characteristics
WBL LIPUS is a low-intensity pulsed ultrasound therapy. Its advantages include:
1.Pulsed design: The low-intensity pulsed ultrasound significantly reduces the thermal effect of continuous ultrasound devices, optimizing mechanical effects.
2.Non-invasive treatment: It requires no invasive procedures, reducing patient discomfort and risk.
Biological Mechanism
LIPUS activates endogenous stem cells to repair endothelial cell function, promotes neovascularization and nerve regeneration, restores the collagen smooth muscle ratio in the penile corpora cavernosa, and inhibits inflammation while alleviating pain. It is a fundamentally non-invasive treatment for erectile dysfunction.
Study Subjects
1.Age ≥ 20 years
2.ED history of at least 6 months
3.No PDE5 inhibitors or other treatments for 4 weeks before starting LIPUS therapy
4.IIEF-EF score ≤ 25 points
5.A stable sexual partner and able to maintain a normal sexual relationship during the study (at least one attempt per week)
Treatment Method
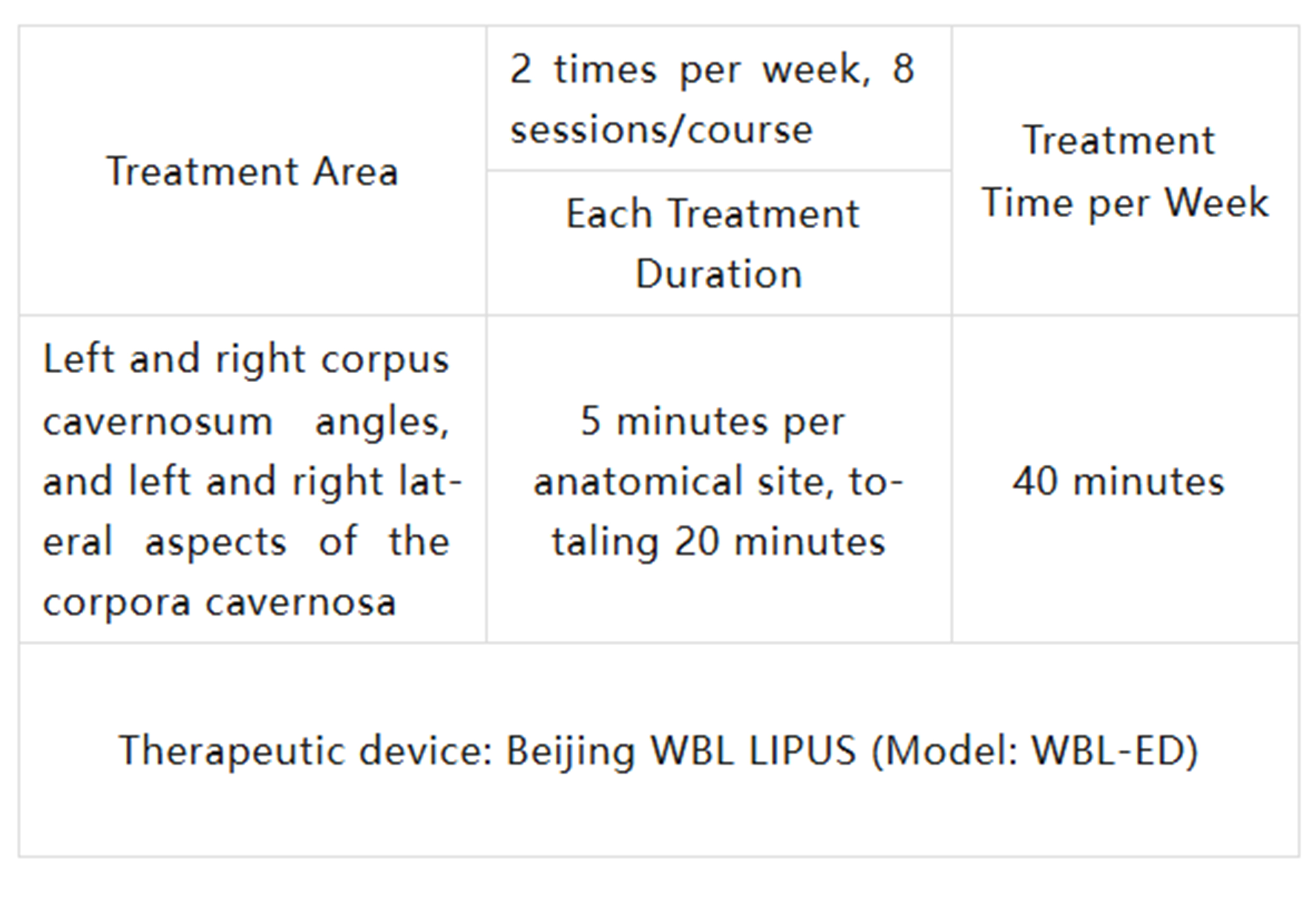
Patients received ultrasound treatment twice a week for 4 weeks, 8 sessions per treatment cycle. The treatment targets four areas on the body.
The treatment time and areas are detailed in the table below:
Observed Indicators
1.LIPUS treatment efficacy is considered effective if the change in IIEF-EF score is greater than the minimal clinically important difference (MCID). For mild, moderate, and severe ED, the MCID is 2, 5, and 7 points, respectively.
2.Other indicators include the EHS rating, SEP questions 2 and 3, GAQ, and penile color Doppler ultrasound (CDDU) measurements of peak systolic velocity (PSV) and end-diastolic velocity (EDV) to assess penile vascular function.
Study Results
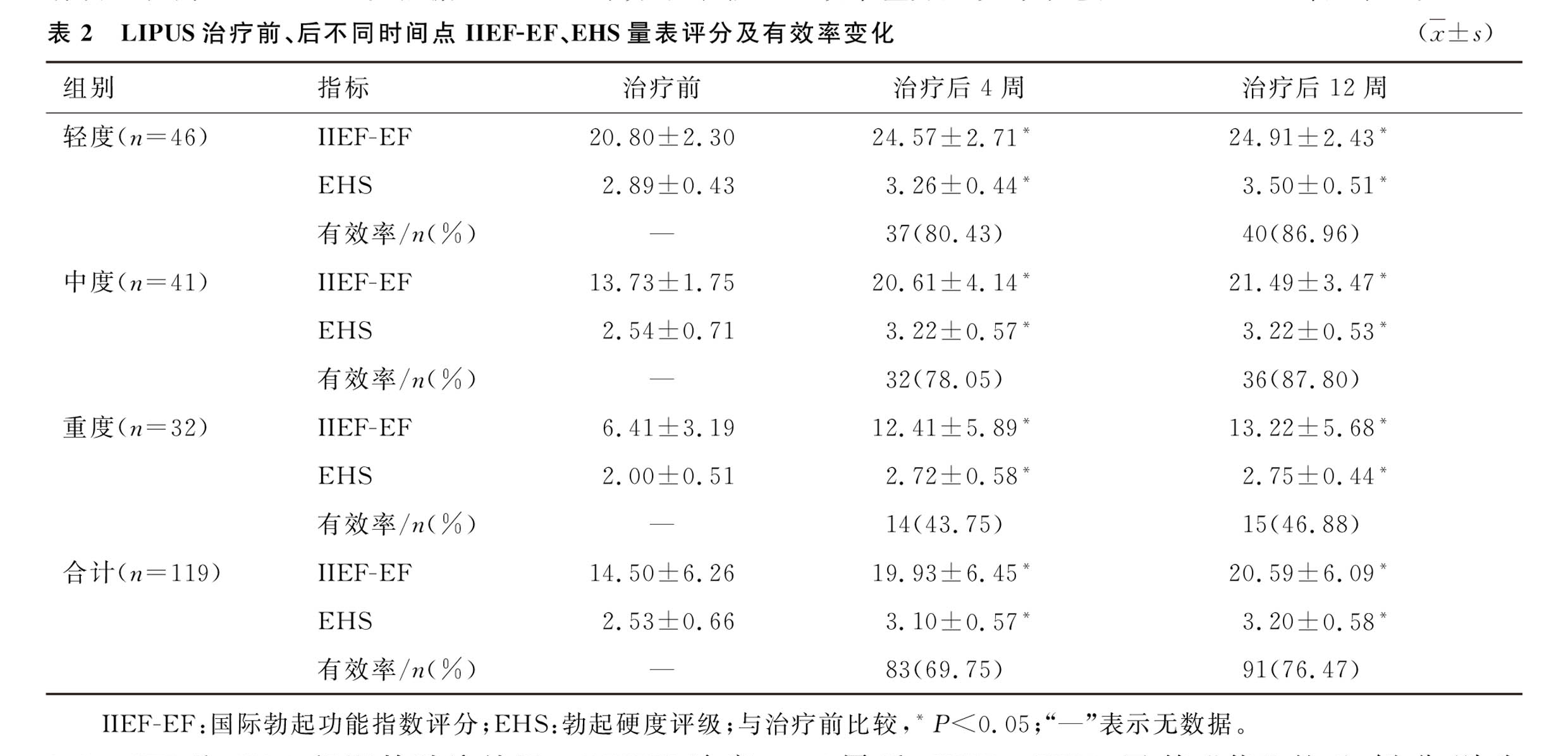
1.IIEF-EF and EHS Scores Follow-Up Results:At the 4th and 12th-week follow-ups, both the IIEF-EF and EHS scores showed improvement compared to baseline. The effective rate was higher in the mild and moderate groups than in the severe group at both the 4-week and 12-week follow-ups, with no significant differences between the mild and moderate groups.
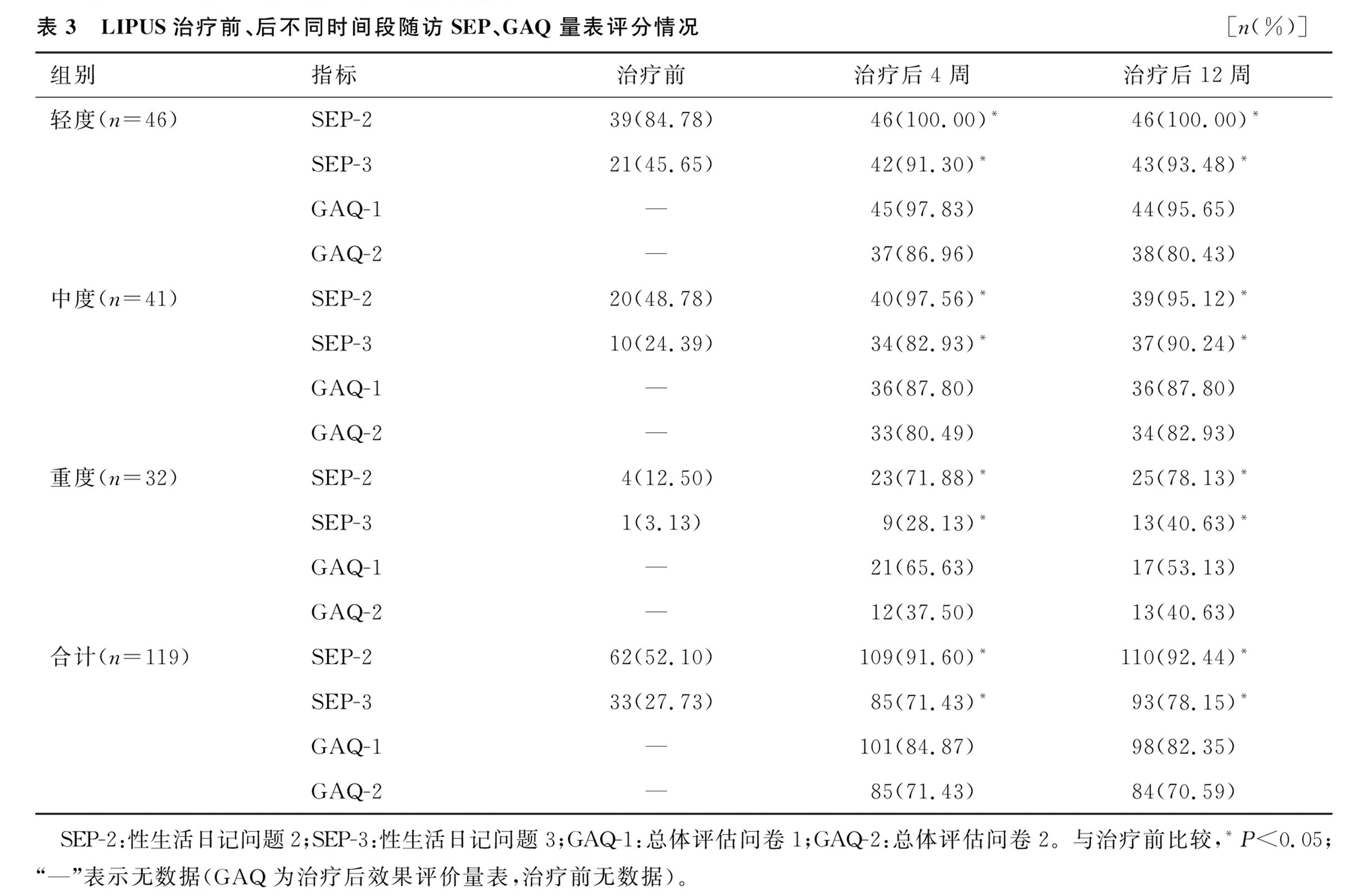
2.SEP and GAQ Follow-Up Results:After 4 weeks of treatment, the percentage of patients answering "yes" to SEP-2 and SEP-3 was 91.60% and 71.43%, respectively. After 12 weeks, these percentages increased to 92.44% and 78.15%, significantly higher than the pre-treatment values (52.10% and 27.73%, P < 0.05). GAQ results after 4 weeks and 12 weeks showed improvements in responses, with no significant difference between the two follow-ups.
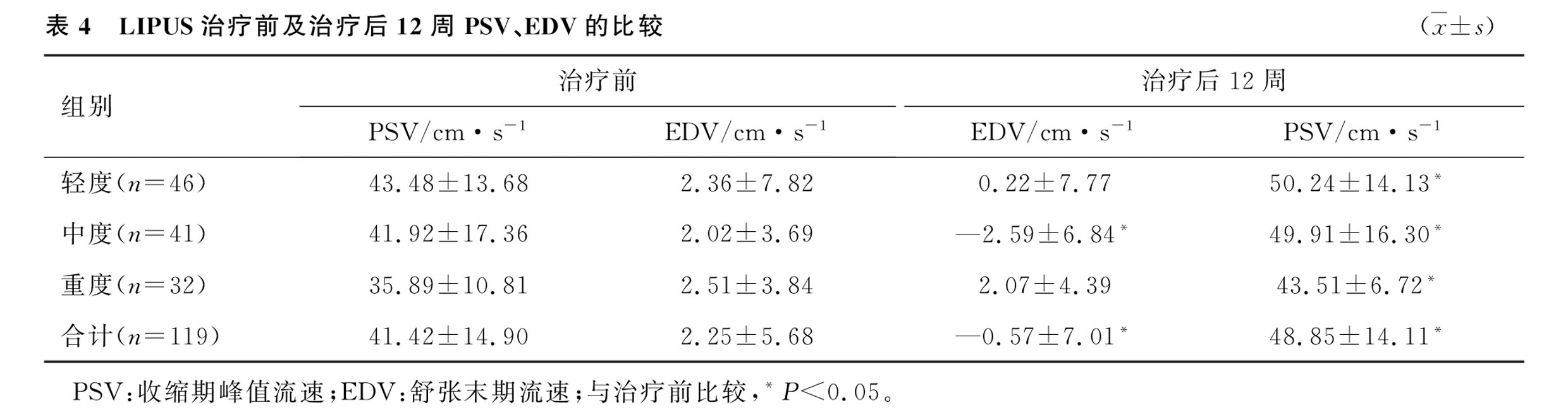
3.PSV and EDV Follow-Up Results:At the 12-week follow-up, PSV levels increased to [(48.85±14.11) cm/s vs. (41.42±14.90) cm/s], a statistically significant difference. EDV levels decreased to [(-0.57±7.01) cm/s vs. (2.25±5.68) cm/s], also showing a significant difference.Throughout the treatment and follow-up periods, no adverse reactions such as local bleeding, purpura, pain, numbness, or tingling occurred.
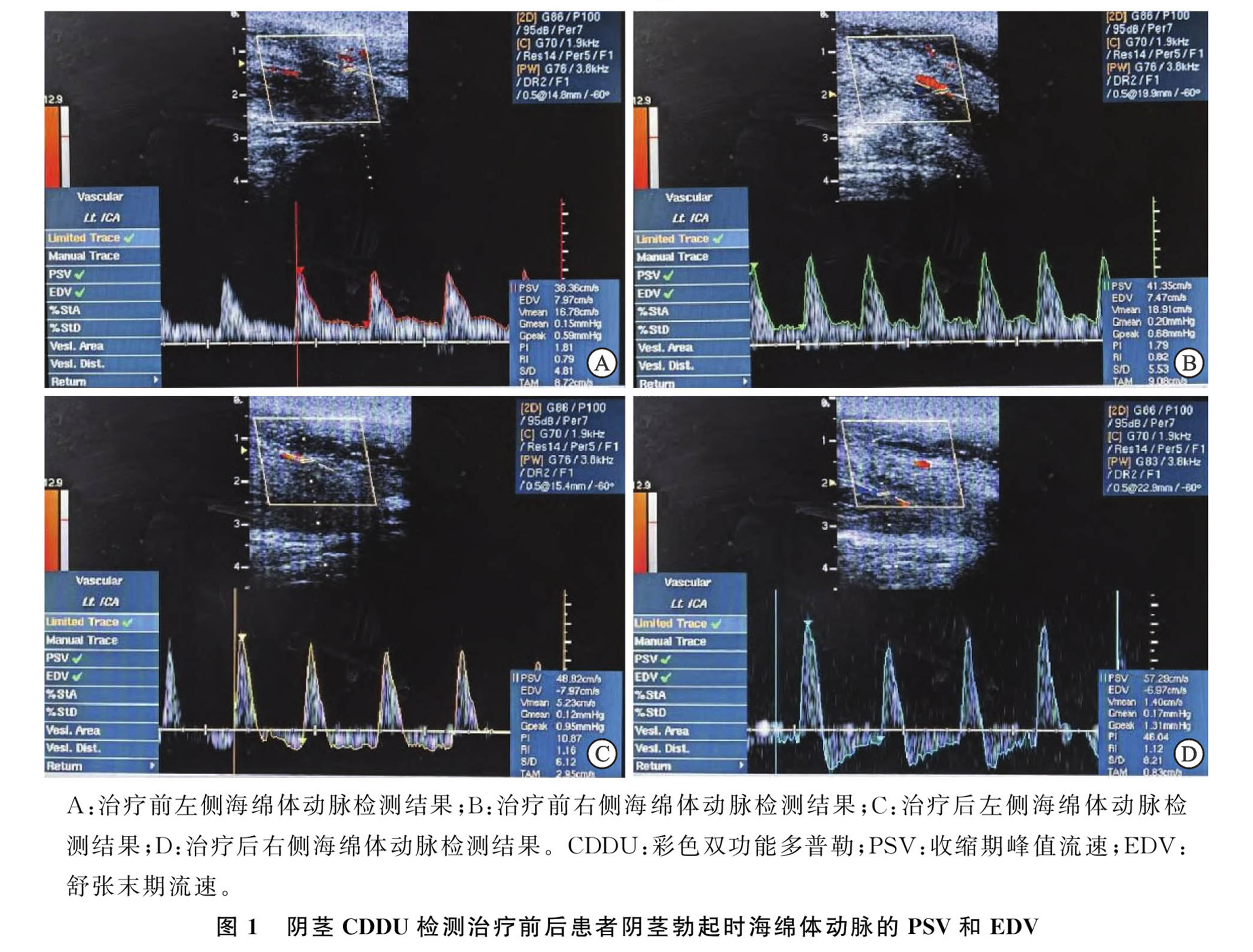
Conclusion
This study demonstrated that LIPUS treatment for ED patients has significant therapeutic effects that last at least 3 months, with no notable adverse reactions, making it suitable for clinical promotion.
The device used in this study was the WBL LIPUS equipment model WBL-ED
WBL Medical Equipment Co., Ltd. is a pioneer in LIPUS technology, and through its independently developed WBL-ED device, it became the first to apply low-intensity pulsed ultrasound technology for treating erectile dysfunction. The device was approved by the China Food and Drug Administration (CFDA) in 2018 and has since filled a technical gap in the field, winning the National Medical Device Innovation Award, reflecting WBL's industry leadership and technical strength.
