Conclusion: Following treatment with WBL Low-Intensity Pulsed Ultrasound (LIPUS), patients demonstrated significant improvements in pain symptom scores and prostate palpation scores on the NIH-CPSI scale. Notable enhancements were also observed in pain symptoms, inflammatory status, urinary symptoms, and quality of life. This study achieved satisfactory clinical efficacy.

References: National Journal of Andrology, Zhonghua Nankexue Zazhi 2025, 31(3): 272-274
In 2025, Xi'an Daxing Hospital and the Xijing Hospital of the Air Force Medical University jointly published an article titled Clinical Observation of Low-Intensity Pulsed Ultrasound in the Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome in the Chinese Journal of Andrology. The study included 105 patients from the urology outpatient department of Xi'an Daxing Hospital affiliated with Yan'an University. Among them, 90 patients completed the treatment and follow-up and were ultimately included in the study, with an average age of (30.32 ± 6.38) years and a mean disease duration of (24.49 ± 13.40) months. This clinical observation was approved by the Ethics Committee (approval number: LW2024-029).
Research Background
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), also termed Type III prostatitis, represents the most prevalent subtype of chronic prostatitis (CP). Clinically, it manifests as persistent pelvic pain or discomfort lasting more than 3 months, frequently accompanied by urinary symptoms (e.g., frequent urination, urgency), sexual dysfunction (including premature ejaculation, erectile dysfunction, and dysorgasmia), and psychological disturbances (anxiety, depression). Owing to perineal pain and inadequate disease awareness, CP/CPPS patients often experience anxiety, depression, and other psychiatric symptoms.
Research Objectives
The majority of CP/CPPS patients endure chronic pelvic pain without any evidence of inflammatory infection. Currently, there is a lack of effective treatment options for CP/CPPS in clinical practice. Therefore, exploring a novel and effective therapeutic approach is particularly important. This study conducted a clinical investigation into the use of low-intensity pulsed ultrasound (LIPUS) for the treatment of CP/CPPS.
Product Features
WBL LIPUS is a low-intensity pulsed ultrasound therapy characterized by intermittent ultrasound emission to minimize thermal effects within safety ranges, significantly enhancing the conversion of mechanical effects into biological effects for disease treatment. The pulsed energy intensity is safe and non-invasive, ensuring convenient and secure usage.
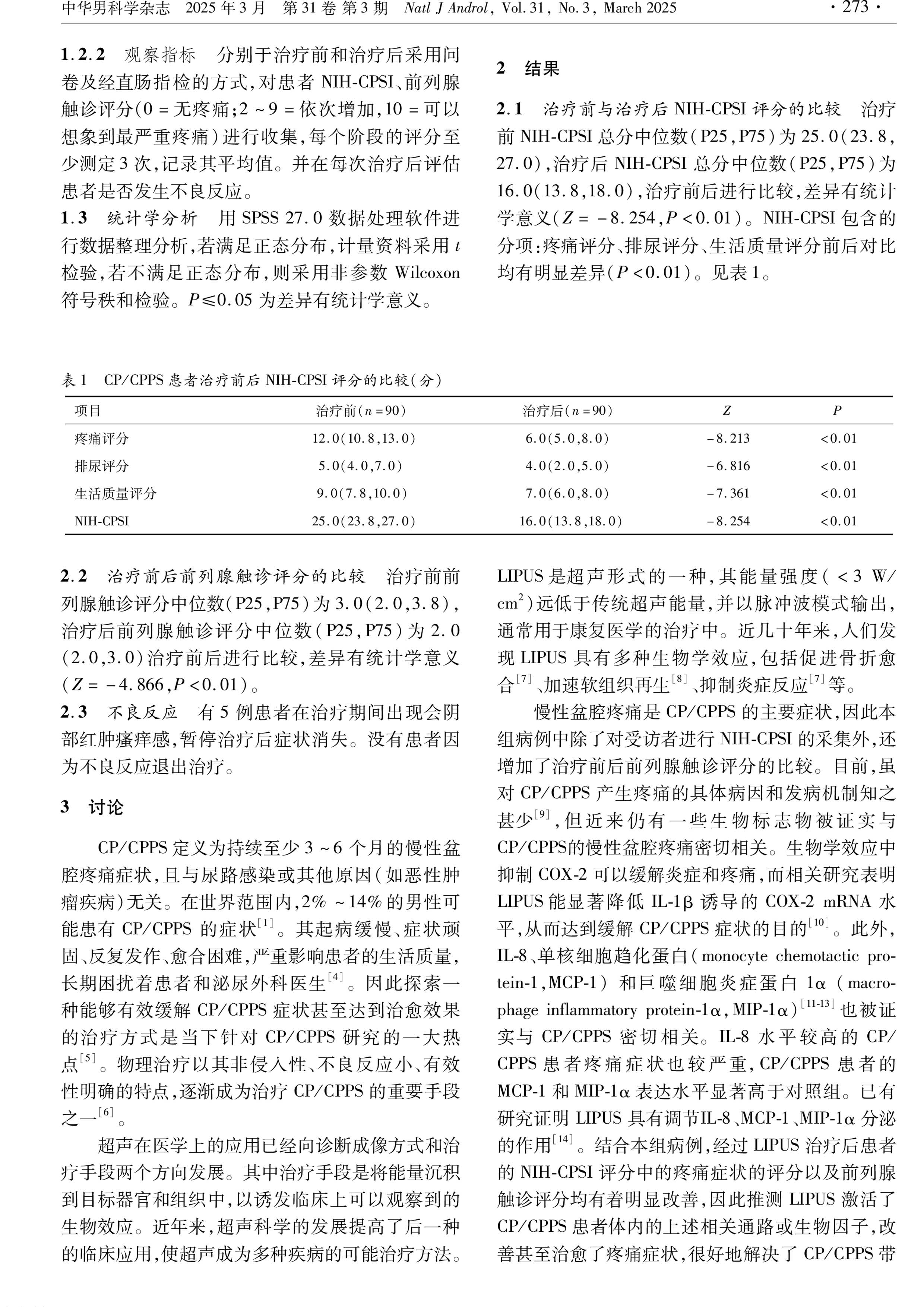
Biological Mechanism
WBL LIPUS alleviates pain and inflammation by activating the TLR4/COX2 pathway and inhibiting the release of oxidative stress and inflammatory factors (such as IL-8, MCP-1). In summary, LIPUS exerts anti-inflammatory, analgesic, and tissue-repairing effects through multiple pathways, including regulating gene expression, cellular signal transduction, angiogenesis, and stem cell differentiation.
Study Population
In accordance with the National Institutes of Health (NIH) classification, eligible patients had long-term recurrent pelvic pain or discomfort lasting over 3 months, negative bacterial cultures from EPS/semen/VB3, an NIH-CPSI pain score ≥ 4, and a first-time diagnosis of CP/CPPS (Type III prostatitis).
Treatment Methods
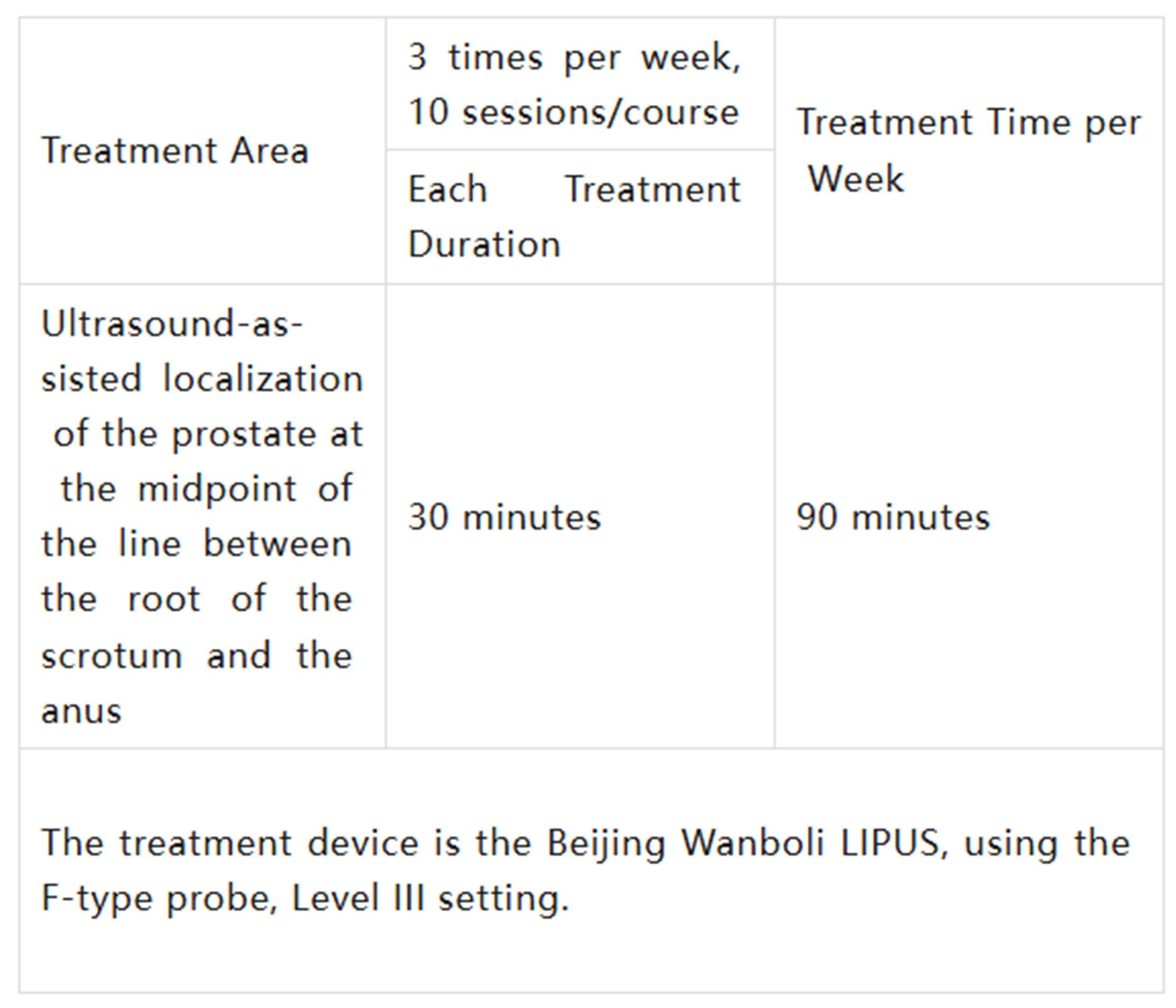
Treatment Site, Contact Method, and Duration:
Patients received ultrasound therapy 3 times weekly for 3 consecutive weeks, totaling 10 sessions per course. Each session involved sequential treatment of one anatomical site.
Detailed treatment times and sites are outlined in the table below:
Observation Indicators
Questionnaires and digital rectal examinations were administered before and after treatment to collect data on patients' NIH-CPSI scores and prostate palpation scores (0 = no pain; 2–9 = increasing severity; 10 = worst imaginable pain). Scores were measured at least three times during each phase, and the average values were recorded. Adverse reactions were assessed after each treatment session.
Research Results
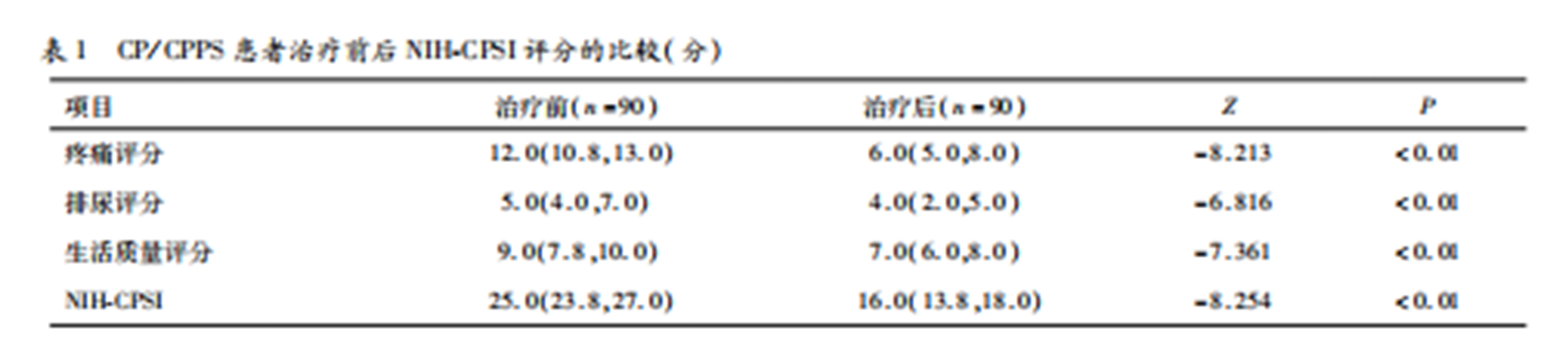
1、Comparison of NIH-CPSI Scores Before and After Treatment
The median NIH-CPSI total score (P25, P75) was 25.0 (23.8, 27.0) before treatment and 16.0 (13.8, 18.0) after treatment, with a statistically significant difference (Z = -8.254, P < 0.01). All NIH-CPSI subscales (pain, urinary symptoms, quality of life) showed significant improvements (P < 0.01). Refer to Table 1.
2、Comparison of Prostate Palpation Scores Before and After Treatment
The median prostate palpation score (P25, P75) was 3.0 (2.0, 3.8) before treatment and 2.0 (2.0, 3.0) after treatment, with a statistically significant difference (Z = -4.866, P < 0.01).
3、Adverse Reactions
Five patients experienced perineal redness, swelling, and itching during treatment, which resolved after temporary discontinuation. No patients withdrew due to adverse reactions.
Conclusion
CP/CPPS, as a chronic condition, often necessitates prolonged treatment. However, patients frequently struggle to adhere to consistent oral or topical medication regimens. During follow-up, LIPUS, as a non-invasive therapeutic modality, was generally well-received by participants due to its treatment comfort. In terms of safety, LIPUS offers advantages such as safety, ease of operation, and high acceptability. Only mild local adverse reactions were observed in this study, with no reports of severe or systemic adverse effects. Thus, LIPUS poses minimal risk to both operators and patients.
The therapeutic device utilized in this trial was the WBL LIPUS model WBL-ED.
WBL Medical Device Co., Ltd. is a pioneer in LIPUS technology. Leveraging its independently developed LIPUS-ED device, the company has become the first to apply low-intensity pulsed ultrasound technology in the treatment of erectile dysfunction, providing a novel, non-invasive, safe, and efficient solution. Since obtaining approval from the China Food and Drug Administration (CFDA) in 2018, WBL LIPUS-ED has not only filled technological gaps in this field both domestically and internationally but has also been honored with the China National Medical Device Innovation Award, underscoring WBL's leading position and technological prowess in the industry.

References: