
WBL Medical Device Co., Ltd. is a pioneer in LIPUS (Low-Intensity Pulsed Ultrasound) technology. With its independently developed LIPUS-ED device, the company was the first to apply low-intensity pulsed ultrasound technology in the treatment of erectile dysfunction (ED), offering a non-invasive, safe, and efficient new solution. Since receiving approval from the China Food and Drug Administration (CFDA) in 2018, the Wanboli LIPUS-ED has not only filled a technological gap in this field both domestically and internationally but also won the China National Medical Device Innovation Award, showcasing WBL leadership and technical strength in the industry.

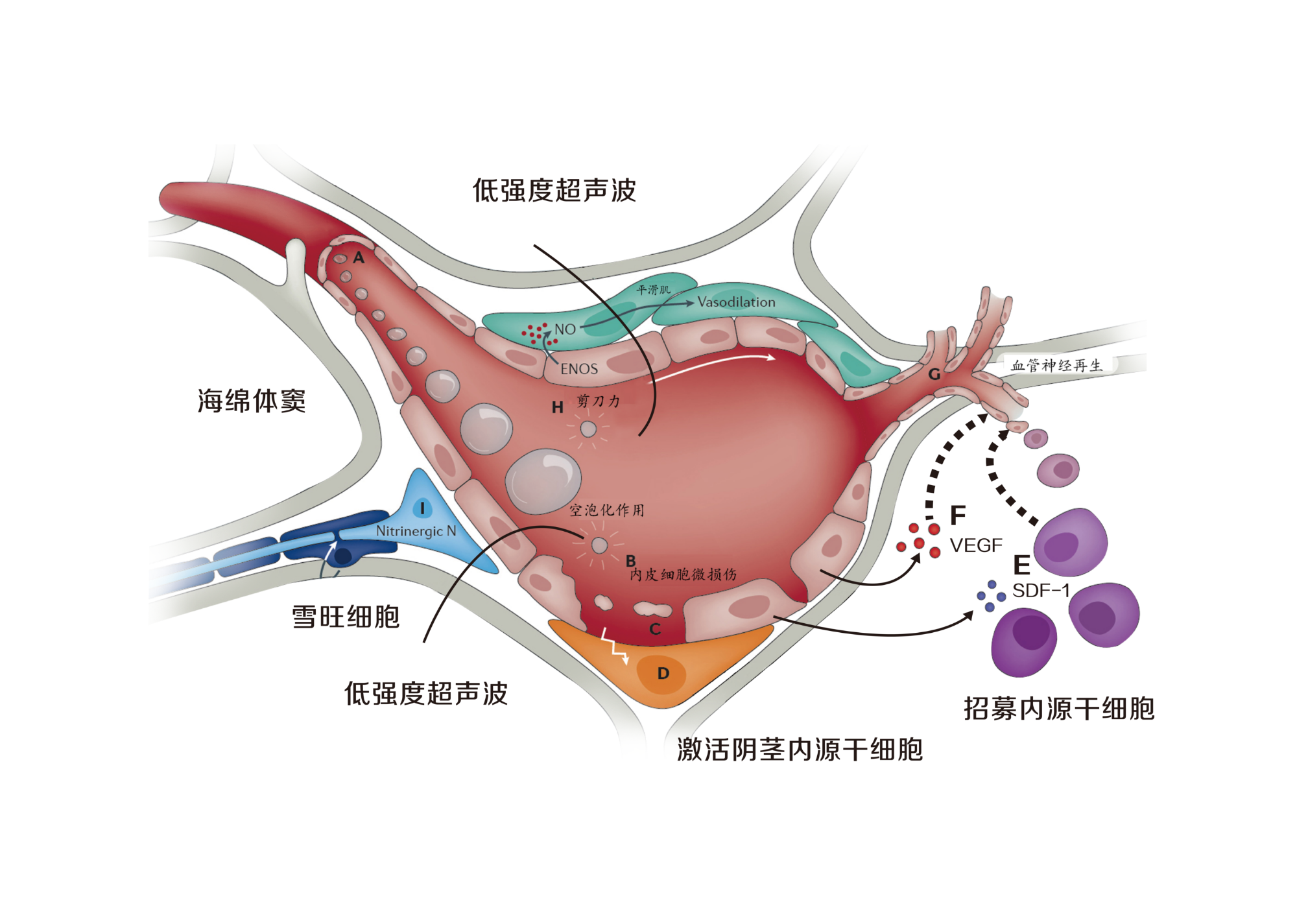
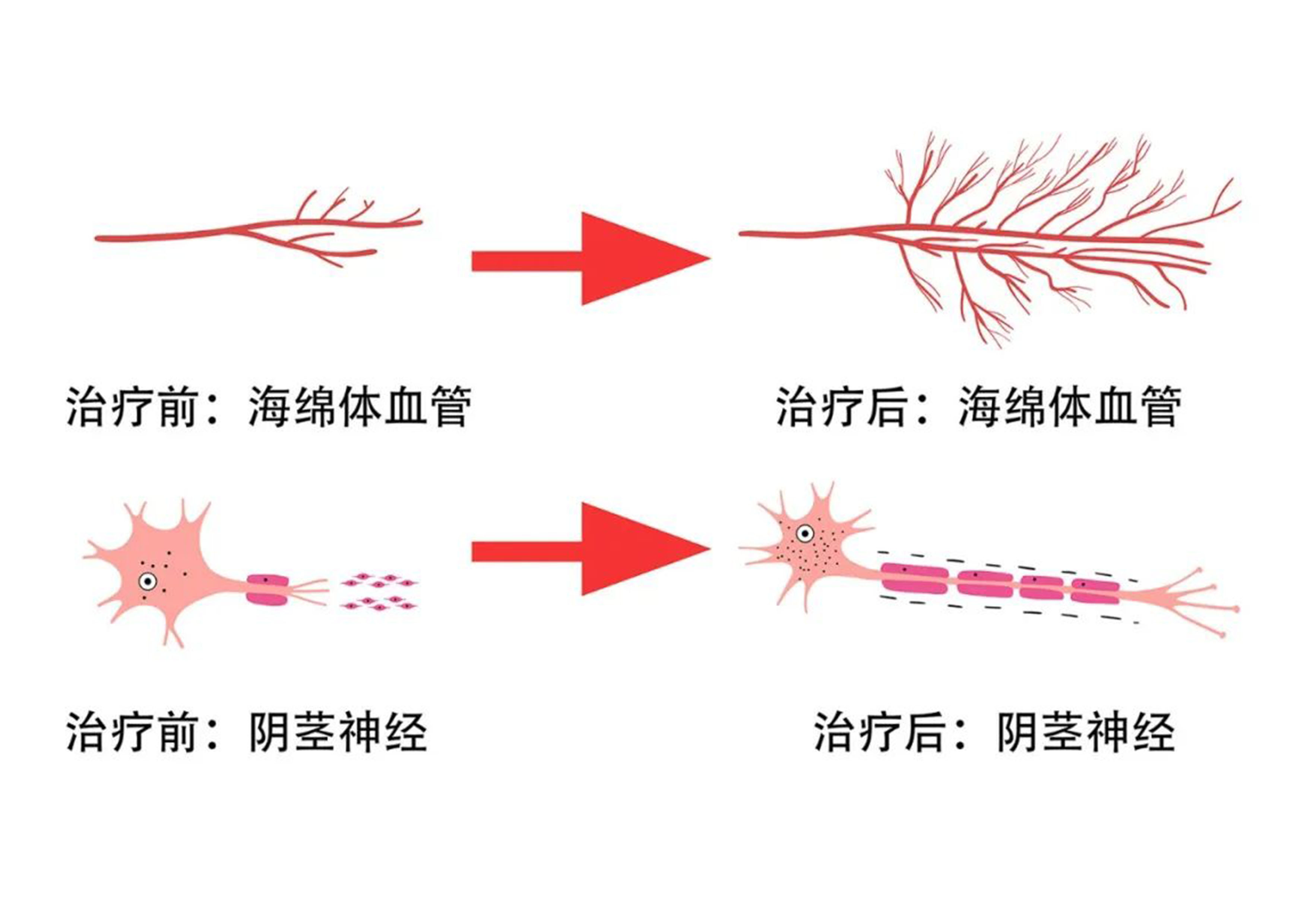
LIPUS generates non-invasive mechanical forces that are converted into biological effects. It regulates the molecular biological signaling pathways in the cell membrane, cytoplasm, and nucleus, promoting blood vessel regeneration, repairing nerve damage, and suppressing inflammation. It repairs pathological changes in the penile corpus cavernosum, including blood vessels, nerves, and matrix, and improves erectile function, which is related to the activation of endogenous stem cells in the penile corpus cavernosum. The mechanical effects of LIPUS activate endogenous stem cells in the penile corpus cavernosum.
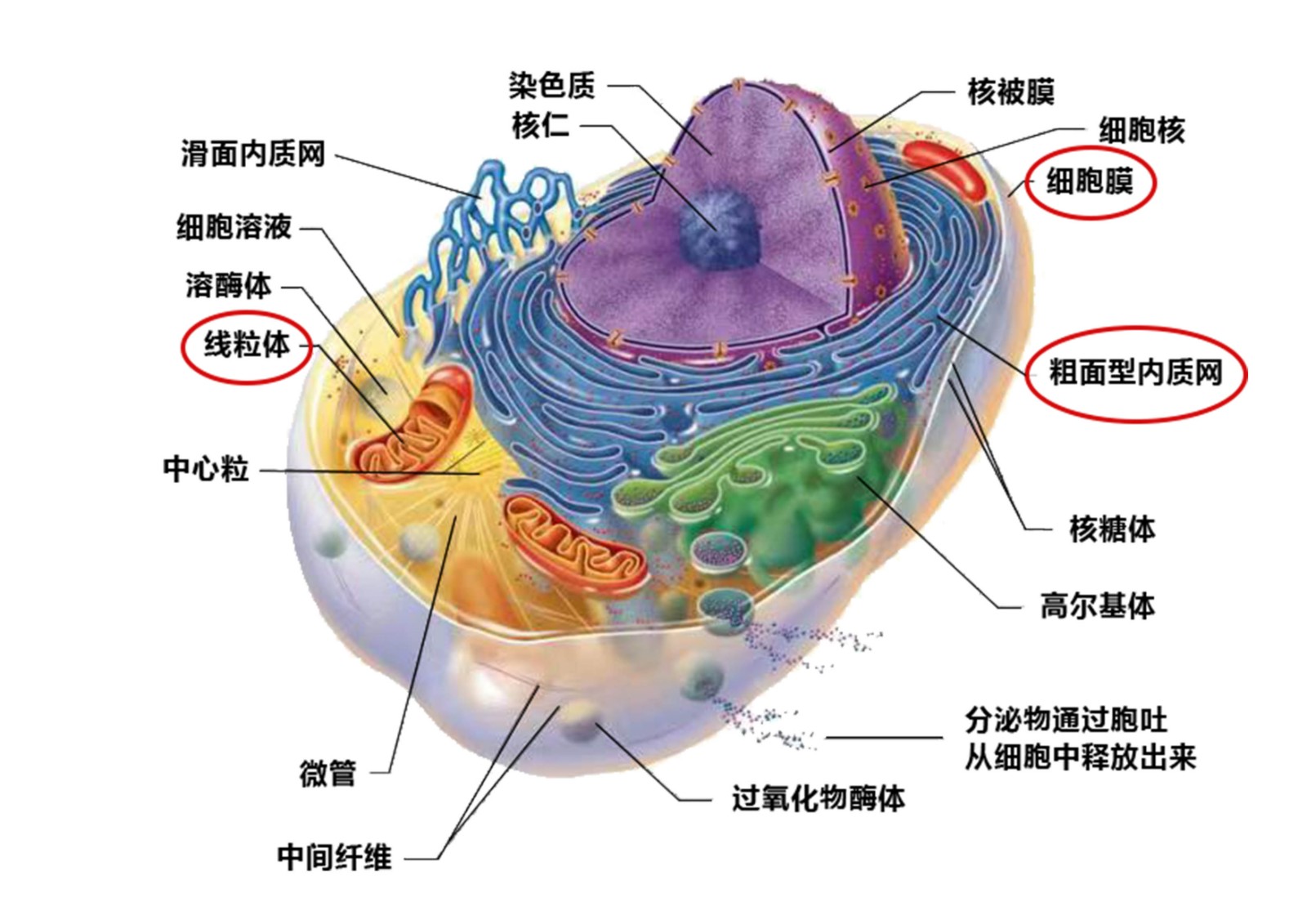
LIPUS mainly affects the cell membrane, mitochondria, and endoplasmic reticulum:
1.It stimulates the mitochondria to produce more ATP, providing energy for the cells.
2.It activates signaling pathways related to cell activation and division.
3.It releases factors associated with vascular and nerve regeneration through these signaling pathways.
Low-Intensity Pulsed Ultrasound (LIPUS) is an innovative, non-invasive technology for the treatment of erectile dysfunction (ED). Compared with traditional ED treatments, LIPUS repairs the pathological changes of ED and promotes the regeneration of nerves and blood vessels, offering a fundamental solution for ED. Unlike regenerative therapies such as growth factor therapy, gene therapy, stem cell therapy, and tissue engineering therapy, LIPUS has been proven to effectively promote nerve and blood vessel regeneration.
Clinical trials show that LIPUS uses a safe ultrasound mechanical force transmission mechanism to activate endogenous stem cells in adult tissue, repairing damaged blood vessels, nerves, and matrix, restoring their normal physiological functions. This mechanism is not only safe and effective but also provides a new treatment option for ED patients.
Development History of Original LIPUS:
2012:
In 2012, the Low-Intensity Ultrasound Treatment for ED project was officially launched, designed by Chinese Academy of Engineering Academician Guo Yinglu and international urology expert Professor Fu-Tai Lu from the University of California, San Francisco.
2013:
In 2013, the finished product developed by Beijing WBL Medical Device Co., Ltd. was successfully delivered to the Male Urology Center of Peking University First Hospital and the UCSF Biological Laboratory for biological mechanism research.
2014:
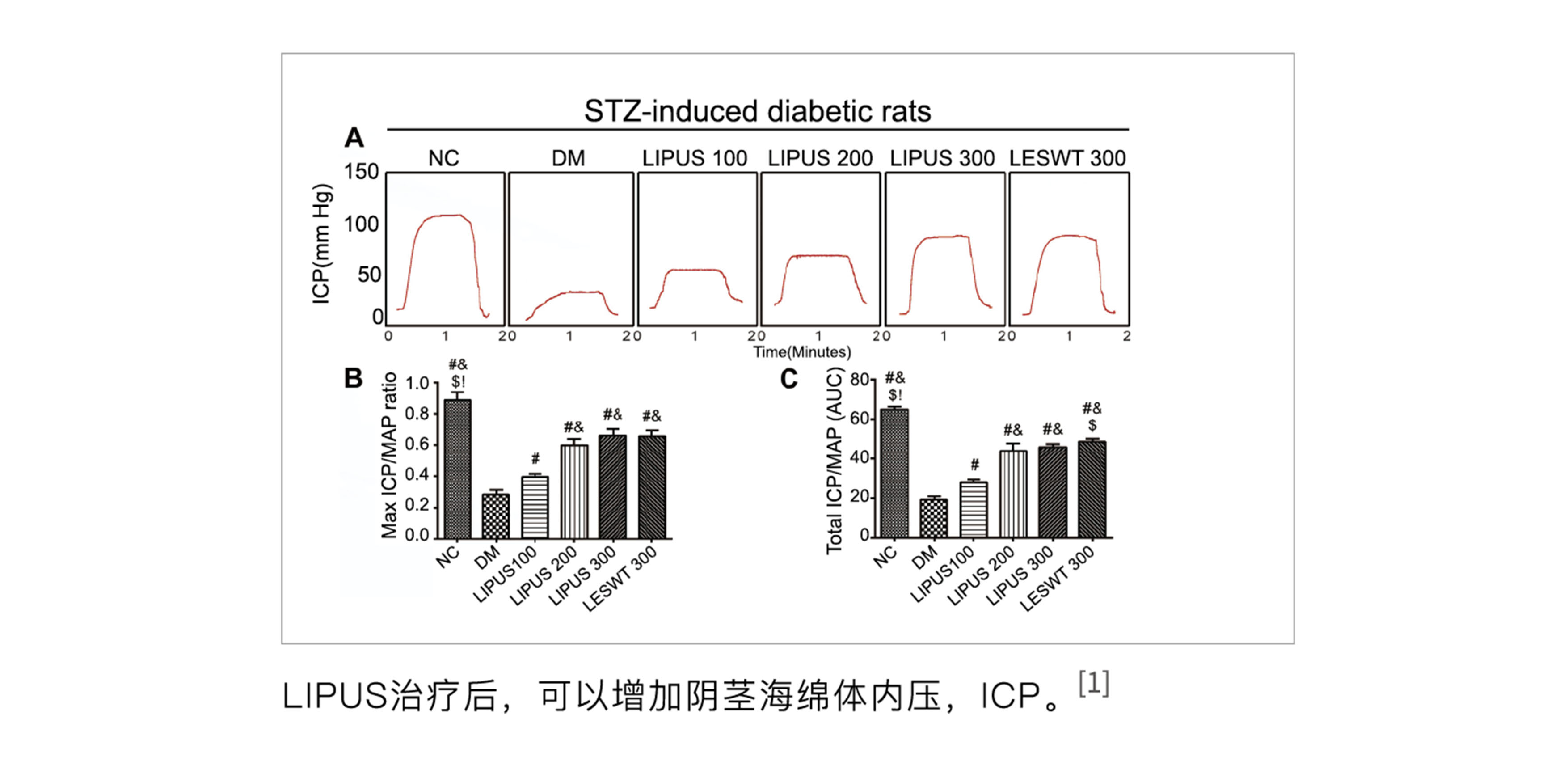
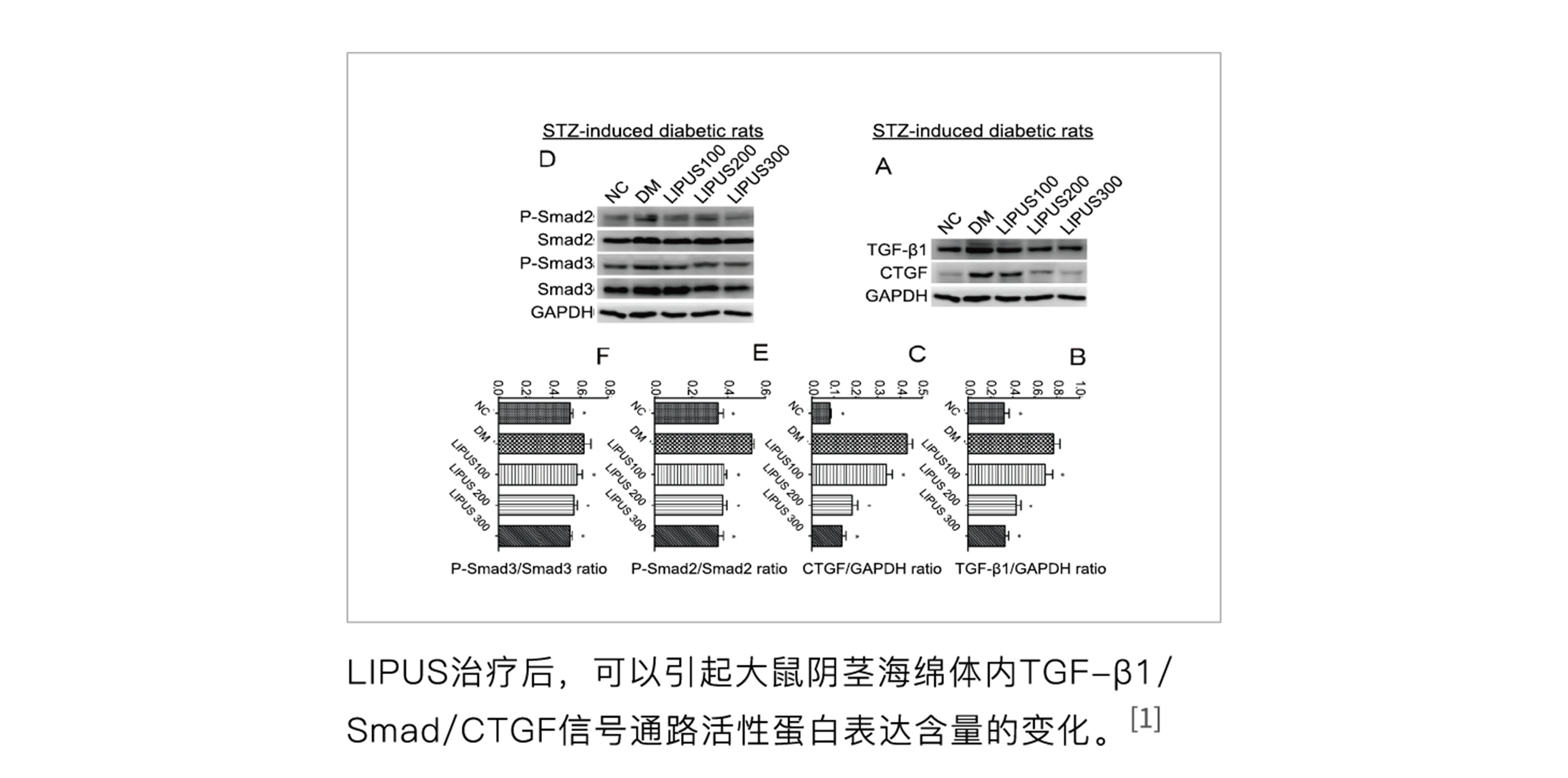
At the end of 2014, the effectiveness of low-intensity ultrasound on STZ-induced erectile dysfunction in rats was verified at Peking University Hospital, providing strong data support for clinical research.
2016:
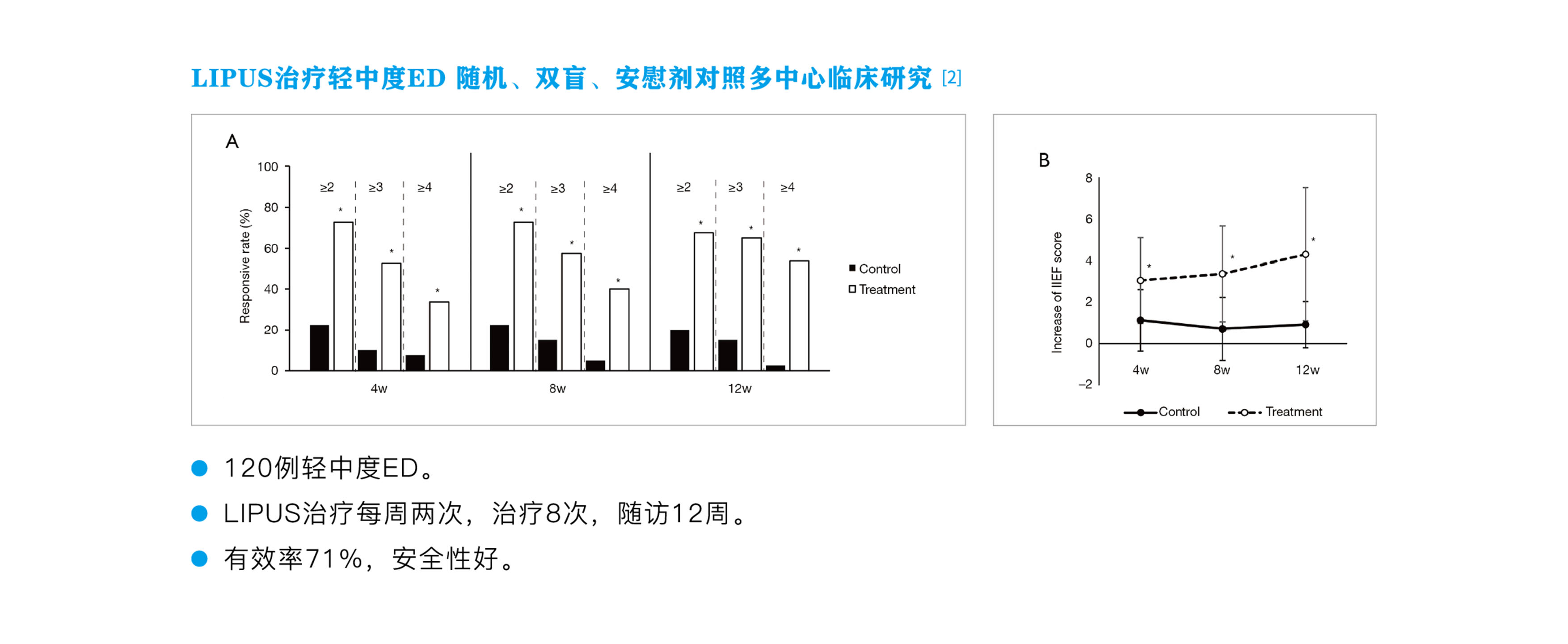
In 2016, the biological mechanism research and animal experiments led by Professors Xin Zhongcheng and Lin Guiting were successfully completed. Additionally, five hospitals conducted clinical trials, treating 120 patients with mild to moderate ED, with an efficacy rate of 71% and good safety.
2017:
In 2017, the Low-Intensity Pulsed Ultrasound Treatment for Erectile Dysfunction technology won the "National Science and Technology Innovation Award," and the project received special approval from the National Medical Products Administration (NMPA).
2018:
In 2018, LIPUS received registration certification from the Food and Drug Administration. In the same year, the Low-Intensity Pulsed Ultrasound Treatment Platform (LIPUS) was launched.
2019:
In 2019, the National Innovative Clinical Trial led by Professor Li Zheng from Shanghai First People's Hospital was launched, studying the effects of LIPUS at different frequencies on ED.
In the same year, the LIPUS Low-Intensity Pulsed Ultrasound ED Treatment Platform received an invention patent certificate.

2020:
In 2020, Professor Xia Shujie from Shanghai Jiao Tong University First People's Hospital presented an academic report on the research progress of mechanical force biological chain-mediated endogenous stem cell activation for the treatment of erectile dysfunction. Research based on the biological matrix of mechanical force was officially established, providing stronger theoretical support for the safe and effective use of Low-Intensity Pulsed Ultrasound in ED treatment. In the same year, LIPUS clinical papers were published in the Chinese Medical Journal.
2021:
In 2021, the number of installed devices surpassed 100 units nationwide.
Reference:
[1] Hongen Lei, Hua Xin, Ruli Guan, Yongde Xu, Huixi Li, Wenjie Tian, Lin Wang, Zhezhu Gao, Yimnglu Guo, Tom F. Lue, Guiting Lin, and Zhongcheng Xin. "Low-intensity Pulsed Ultrasound Improves Erectile Function in Streptozotocin-induced Type I Diabetic Rats," Urology, 86(6), 2015.
[2] Cui W, Li H, Guan R, et al. "Efficacy and Safety of Novel Low-Intensity Pulsed Ultrasound (LIPUS) in Treating Mild to Moderate Erectile Dysfunction: A Multicenter, Randomized, Double-Blind, Sham-Controlled Clinical Study," Transl Androl Urol, 2019; 8(4): 307-319.
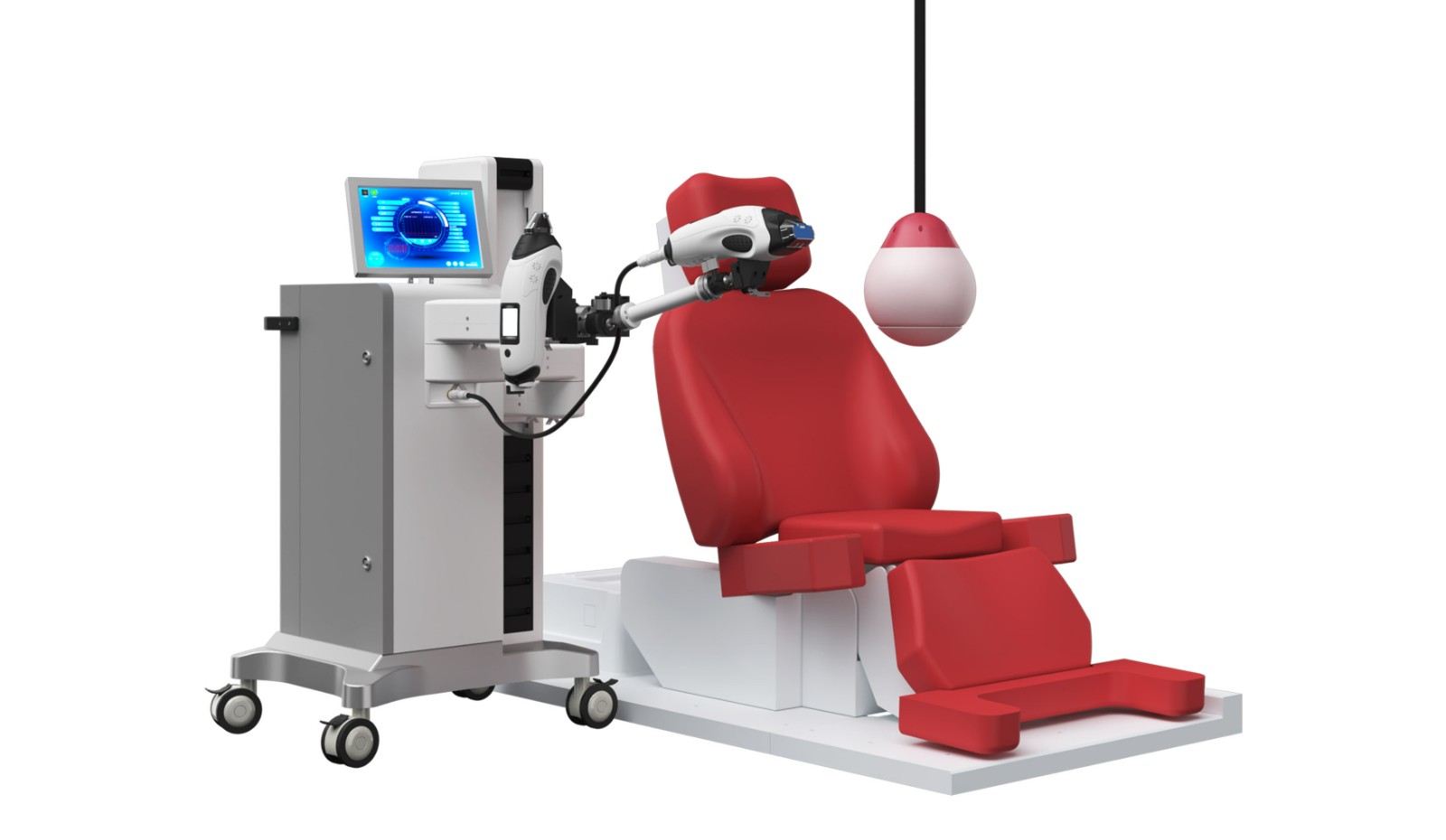
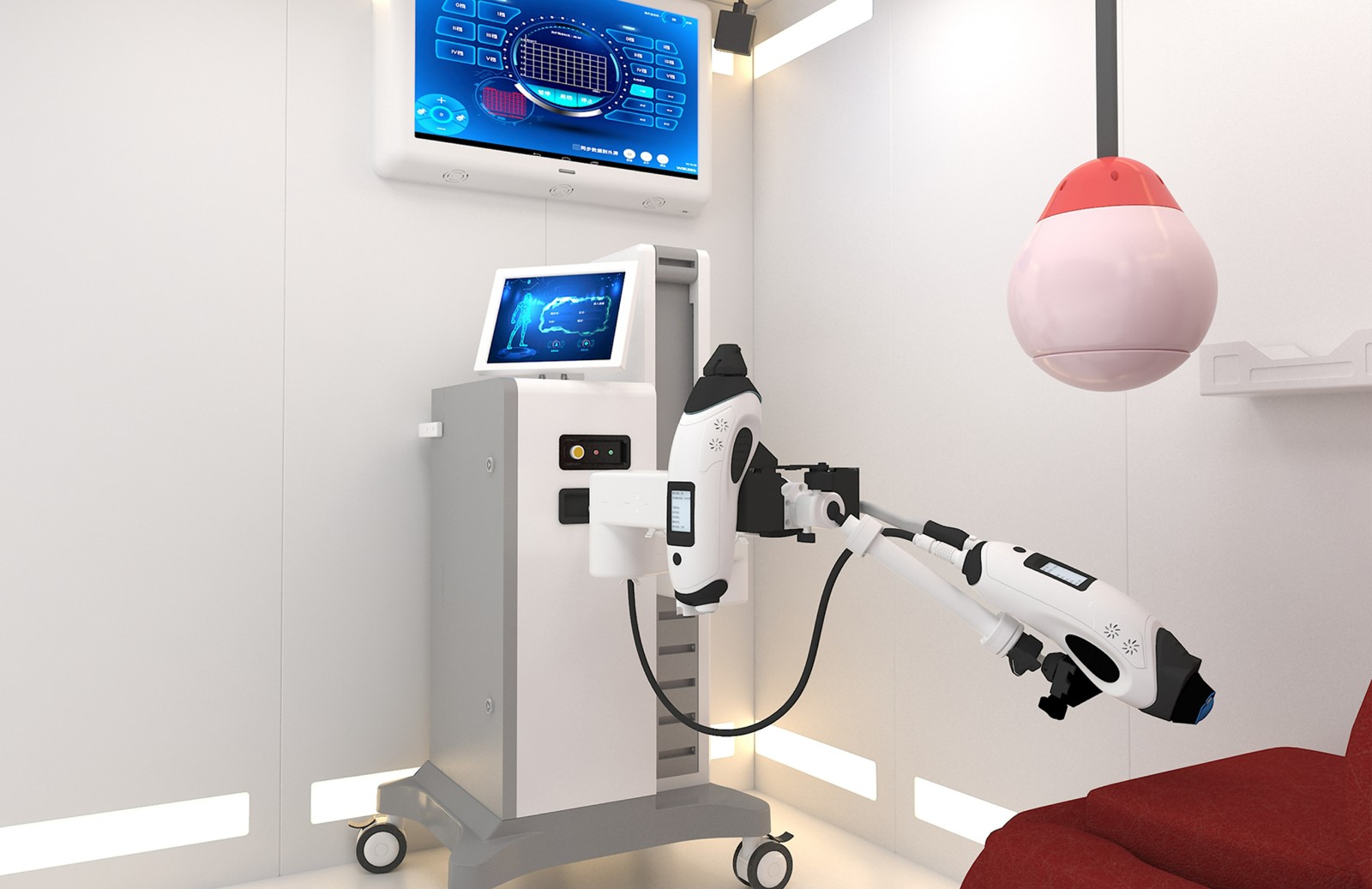
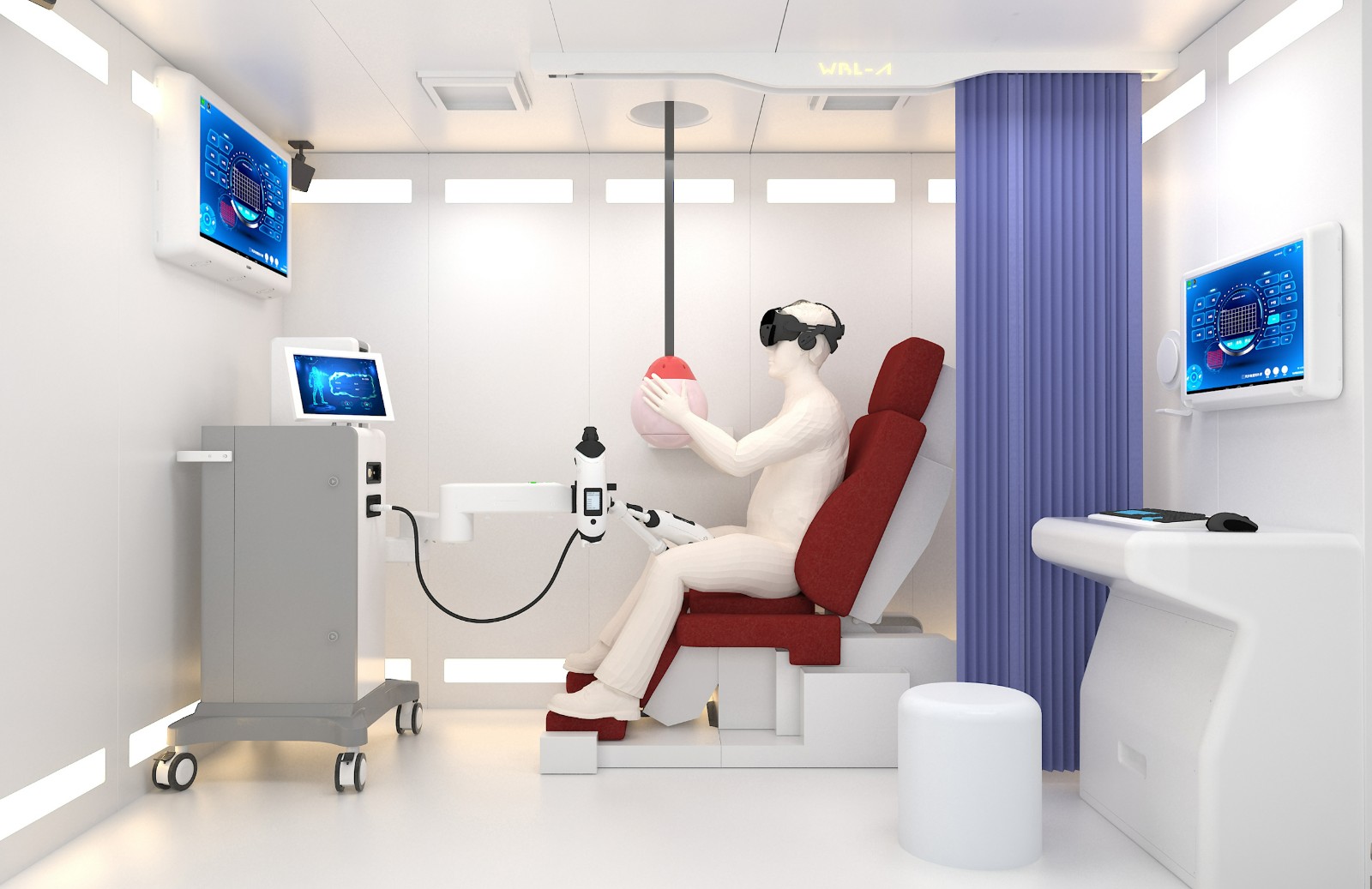
LIPUS is an innovative treatment platform that integrates psychological and physiological treatment, providing a unique global solution. Its core feature is the sex education and psychological counseling system, which includes four sets of sex education videos and four sessions of sex arousal virtual reality experiences. By combining education with immersive technology, it helps users deepen their understanding of sex. Additionally, users can enjoy one-on-one consultations with professional medical staff, focusing on resolving psychological factors related to erectile dysfunction (ED).
The LIPUS platform is also equipped with advanced cavernosal treatment probes, utilizing F-type and Y-type modes, with a maximum power of 3.5W. The treatment position accurately covers the left and right corpora cavernosa and penile roots. Through personalized treatment plans, it is recommended to undergo 2 to 3 treatments per week to effectively improve the blood flow in the penile corpus cavernosum.
With its professional counseling system and advanced treatment technology, the LIPUS platform provides users with a comprehensive solution to enhance erectile function overall.
