

On the second day of the MEDICA exhibition, WBL's booth continues to attract global attention, with physicians, distributors, and research representatives from Europe, the Middle East, and the Asia-Pacific region gathering together. Unlike the preliminary exchanges on the first day, today’s focus shifted to in-depth technical inquiries about the products and substantial discussions on cooperation models. The endorsement from international authoritative experts further enhanced the efficiency of the collaboration process, propelling WBL's global cooperation with LIPUS micro-energy medicine into the fast lane.






Key Highlights: International Urology Expert Visits, Professional Endorsement Attracts Attention

The most influential moment at the booth today was the visit of Prof. Dr. Tayyar Alp Özkan, M.D., FEBU, an international authority in urology from Turkey. Prof. Alp is not only a renowned academic but also a clinical user of LIPUS, which makes his presentation particularly eye-catching.
Prof. Alp holds numerous prestigious professional titles, including membership in the European Association of Urology (EAU), the American Urological Association (AUA), fellow of the European Board of Urology (FEBU), examiner of the European Urology Examination Board (EBU), board member of the Turkish Urological Society (2019–2025), and founder of the UroCaD academic database. Standing at the booth,
Prof. Alp engaged in lengthy technical exchanges with physicians from multiple countries, analyzing the true value of WBL LIPUS from his unique perspective as both a physician and a user.

Prof. Alp shared detailed insights on the treatment parameters and case feedback for ED (Erectile Dysfunction) and chronic prostatitis, comparing the differences between LIPUS and ESWT (Shockwave Therapy) in terms of pain, energy transmission, and biological effects. He also shared the recovery trajectories of patients and his own experience with LIPUS treatment. These first-hand experiences provided the attending physicians and distributors with a clearer and deeper understanding of the clinical value of LIPUS, earning widespread recognition.

During the discussion, two professional interactions were particularly noteworthy. With a distributor from the Czech Republic, Prof. Alp compared the indications and energy mechanisms of LIPUS and ESWT, providing valuable reference for precise market positioning. With an Irish physician, he discussed the introduction methods and clinical processes in men's health care, exploring optimized treatment plans together.

Prof. Alp's international reputation also gained the trust of many European physicians. After learning about his background, several physicians returned to the booth for further clinical information. Prof. Alp patiently explained why WBL LIPUS represents the next generation of non-invasive treatments, which types of patients are best suited for this therapy, how to establish LIPUS treatment pathways in clinics or hospitals, and how to design traceable treatment courses. His explanations provided clear directions for the clinical application of LIPUS and filled the physicians with confidence in this innovative technology.
Furthermore, Prof. Alp offered important suggestions for WBL's international development. He emphasized that after obtaining CE certification, WBL should quickly launch multi-center clinical studies, with the long-term goal of incorporating LIPUS into the EAU's ED treatment guidelines. Currently, EAU includes ESWT, and LIPUS, with its advantages of safety, pain-free, and repeatable treatments, has the potential to be included in these guidelines. This advice set a strategic direction for WBL’s future development.
Multinational Cooperation: Parallel Tracks for Clinical and Market Expansion, Together Building a Global Presence
In addition to in-depth exchanges with international experts, today's booth also welcomed distributors from various countries. They were no longer satisfied with simple product inquiries but actively engaged in discussions about “clinical + market” cooperation, jointly exploring the promotion paths for LIPUS in their local markets.
1.Vietnam: Completing the Southeast Asia Puzzle, Forum + Demonstration Center Double Strategy
A major medical group from Vietnam held in-depth discussions with WBL, reaching multiple cooperation intentions. They plan to host the Vietnam LIPUS Medical Forum in 2026, bringing together renowned urologists from both northern and southern regions to explore the application prospects of LIPUS in the Vietnamese medical field. Additionally, a LIPUS demonstration center will be set up to provide on-site training and operational guidance for local medical personnel. They also explored whether they could accelerate the approval process in Vietnam through Malaysia's MDA registration and arranged in-hospital demonstrations and cooperation talks for 2026. Vietnam's inclusion completes the Southeast Asian market map (Thailand / Malaysia / Indonesia / Vietnam), laying a solid foundation for the promotion of LIPUS in the region.


2.Czech Republic: 30 Years of Medical Enterprise Building Technological Cooperation, Creating European Demonstration
A Czech enterprise with 30 years of experience in medical business highly recognized LIPUS after experiencing it. As a long-time distributor of ESWT, they keenly identified the market potential of LIPUS. The discussions focused on comparing LIPUS and ESWT, highlighting LIPUS's advantages through direct demonstrations; conducting urology case series research to accumulate clinical data; introducing LIPUS into hospitals in the Czech Republic and Slovakia to expand product influence; and jointly participating in the 2026 European Urology Annual Meeting to enhance brand recognition. The Czech Republic, with its high level of medical professionalism, is an ideal European demonstration country. This collaboration will provide strong support for LIPUS's promotion in the European market.


3.Switzerland: Clinical Cooperation Officially Launched, Distributors Follow Up
On November 16th, WBL held an in-depth professional meeting with renowned Swiss urology professor Alex Bachmann in Düsseldorf to officially launch clinical cooperation in Switzerland. They discussed various aspects such as the complete clinical operation of LIPUS C, ED/CPPS treatment parameters, PRP+LIPUS regenerative medicine applications, Y-probe treatment for Peyronie's Disease (PD), male/female UI and intimate medicine applications, Switzerland's PD reimbursement system, and cooperation structure for entering the Swiss market. This collaboration will provide professional guidance for the clinical application of LIPUS in Switzerland and pave the way for its market entry.


The Swiss distributor who returned today already knew Prof. Alex and, upon learning that WBL had officially initiated clinical cooperation with him, immediately expressed strong interest in cooperation. They expressed their desire to follow up on the clinical developments and discuss the Swiss market cooperation model, believing that WBL LIPUS is a more promising solution than shockwave therapy. They emphasized, "I was already looking for a new ED solution, and after seeing WBL LIPUS, I think it's better suited to our market needs." This positive feedback has created a complete structure for WBL in Switzerland, combining "clinical validation + distributor introduction," with high strategic value for both Central and Eastern European markets.

WBL LIPUS Technology Principles and Treatment Effects
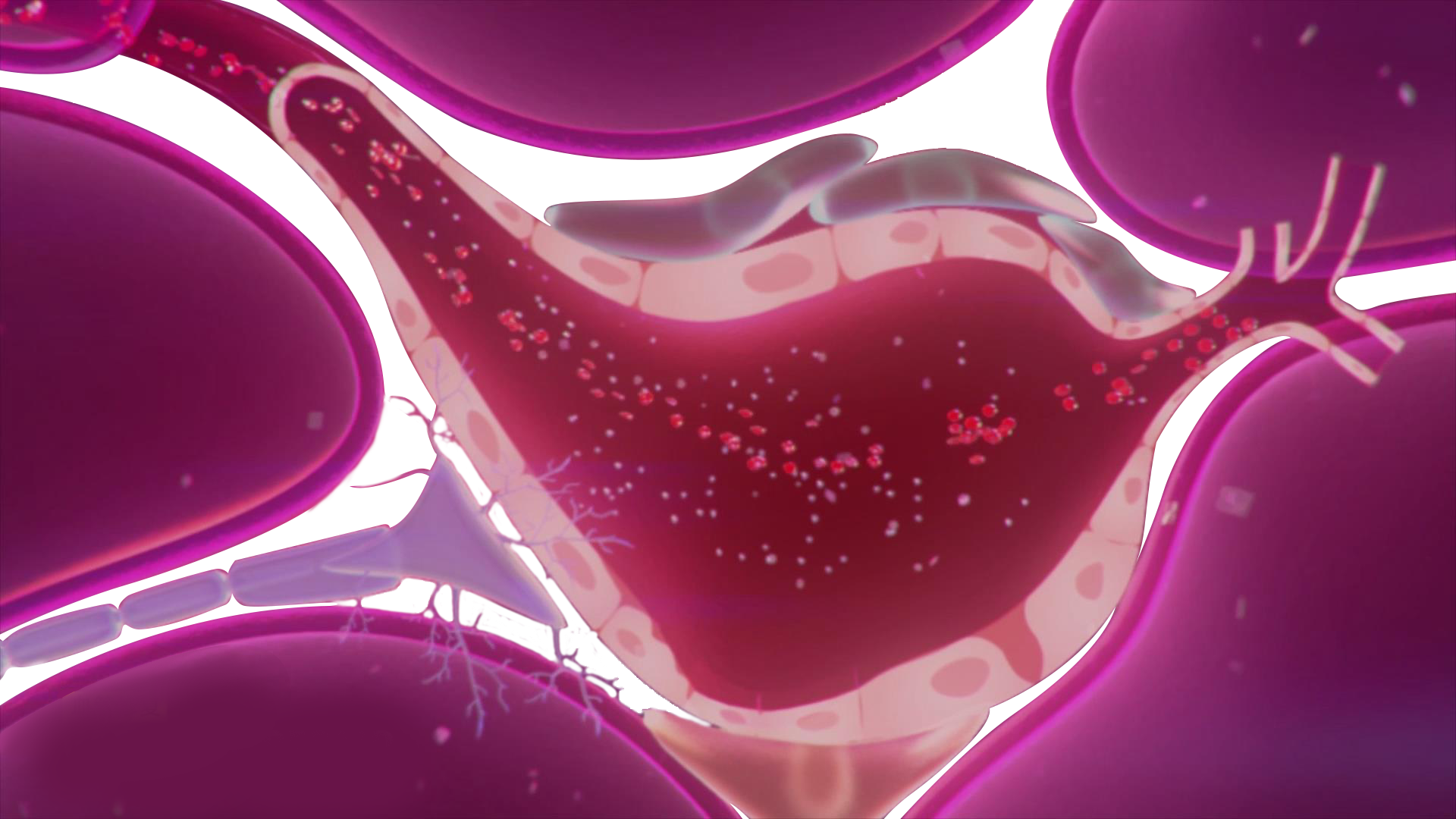
The core mechanism of WBL LIPUS is to use low-energy pulsed ultrasound (intensity range 50-350 mW/cm²) to act on the penile erectile tissue, activate endogenous stem cells, promote vascular and nerve regeneration, repair damaged vascular endothelium and nerve matrix, thus improving erectile function at the pathological level. Compared to traditional oral medications (such as PDE5 inhibitors), prosthetic implants, or invasive therapies (such as intracavernous injections), WBL LIPUS offers a safe, non-invasive treatment that does not cause drug dependency, related side effects, or surgery-related complications.
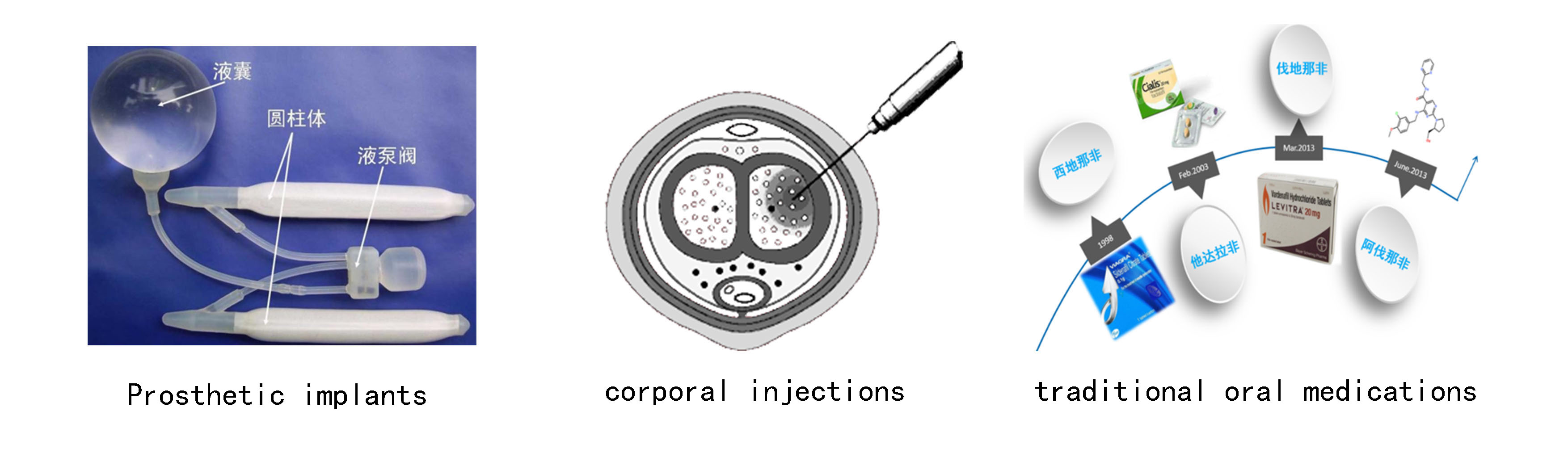
Product Treatment Process and Operation Guidelines
1.Non-invasive and Safe:
No drugs or surgery required, avoiding liver and kidney burden, and reducing infection risks, especially suitable for patients with chronic conditions (e.g., diabetes).
2.Comprehensive Treatment Model:
The unique "LIPUS + psychological counseling + VR" integrated treatment method, which addresses both physiological repair and psychological intervention, improves patient satisfaction by over 30%.
3.Clinical Data Support:
Clinical validation from over 60 medical institutions worldwide, with efficacy and safety backed by authoritative institutions such as UCSF and Peking University Hospital.
From Innovative Technology to Global Standards, Leading the New Wave of Non-Invasive Treatments
The second day of the MEDICA exhibition has been significant for WBL. It is no longer just a platform for showcasing products but has become an important opportunity for multinational KOLs to initiate clinical cooperation, for distributors to discuss demonstration centers and market introductions, and for international experts to provide treatment process and product upgrade suggestions. Cooperation assessments have been initiated simultaneously in Switzerland, the Czech Republic, and Vietnam, marking the shift of LIPUS from an “innovative medical technology” to the path of becoming a "global standard for the next generation of non-invasive treatments."
With deepening international cooperation, WBL micro-energy medicine is poised to lead a revolution in non-invasive treatments worldwide, bringing safe and effective solutions to more patients and opening a new chapter in healthcare.